Financial IncEntives for Smoking TreAtment: protocol of the FIESTA trial and FIESTA Oral Microbiome Substudy
- PMID: 30463608
- PMCID: PMC6249787
- DOI: 10.1186/s13063-018-3003-y
Financial IncEntives for Smoking TreAtment: protocol of the FIESTA trial and FIESTA Oral Microbiome Substudy
Abstract
Background: Smoking is the leading preventable cause of death in the United States, but evidence-based smoking cessation therapy is underutilized. Financial incentive strategies represent an innovative approach for increasing the use of counseling and pharmacotherapy. If effective, they could supplement or supplant resource-intensive policy options, particularly in populations for whom smoking has substantial societal costs. FIESTA (Financial IncEntives for Smoking TreAtment) will randomize hospitalized smokers to receive usual smoking cessation care alone or usual smoking care augmented with financial incentives. We aim to compare the impact of these two strategies on 1) smoking abstinence, 2) use of counseling and nicotine replacement therapy, and 3) quality of life of participants. We also will evaluate the short-term and long-term return on the investment of incentives. The FIESTA Oral Microbiome Substudy will compare the oral microbiome of smokers and nonsmokers to longitudinally assess whether smoking cessation changes oral microbiome composition.
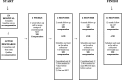
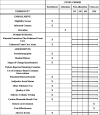
Methods: We will enroll 182 inpatient participants from the Manhattan campus of the Veterans Affairs New York Harbor Healthcare System. All participants receive enhanced usual care, including screening for tobacco use, counseling while hospitalized, access to nicotine replacement therapy, and referral to a state Quitline. Patients in the financial incentive arm receive enhanced usual care and up to $550 for participating in the New York Smoker's Quitline, using nicotine replacement therapy (NRT), and achieving biochemically confirmed smoking cessation at 2 months and 6 months. In the microbiome substudy, we enroll nonsmoking control participants matched to each recruited smoker's hospital ward, sex, age, diabetes status, and antibiotic use. After discharge, participants are asked to complete periodic phone interviews at 2 weeks, 2 months, 6 months, and 12 months and provide expired carbon monoxide and saliva samples at 2 months, 6 months, and 12 months for cotinine testing and oral microbiome analysis.
Discussion: The incentive interventions of FIESTA may benefit hospitalized smokers, an objective made all the more critical because smoking rates among hospitalized patients are higher than those in the general population. Moreover, the focus of FIESTA on evidence-based therapy and bioconfirmed smoking cessation can help guide policy efforts to reduce smoking-related healthcare costs in populations with high rates of tobacco use and costly illnesses.
Trial registration: ClinicalTrials.gov, NCT02506829 . Registered on 1 July 2014.
Keywords: FIESTA; Financial incentives; Manhattan VA Hospital; Smoking cessation; Veterans.
Conflict of interest statement
Ethics approval and consent to participate
The protocol was approved by the Institutional Review Board of the Manhattan Campus of the VA NY Harbor Healthcare System, protocol no. 01494. The principal investigators and research staff are responsible for obtaining informed consent from all study participants. Protocol amendments or amendments to informed consent forms are sent to the Institutional Review Board for approval. Access to data with participants’ protected health information is limited to the investigators, research staff, and the Institutional Review Board. The full protocol may be requested by contacting the principal investigators.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Adhikari B, Kahende J, Malarcher A, Pechacek T, VT Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR. 2008;57(45):1226–1228. - PubMed
-
- Malarcher A, Dube S, Shaw L, Babb S, Kaufmann R. Quitting smoking among adults—United States, 2001–2010. MMWR. 2011;60(44):1513–1519. - PubMed
-
- Fiore M, Jaen CR, Baker T, et al. Treating tobacco use and dependence: 2008 update. Rockville: US Department of Health and Human Services; 2008.

