Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice
- PMID: 30463610
- PMCID: PMC6249754
- DOI: 10.1186/s13287-018-1065-0
Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice
Abstract
Background: Spermatogonial stem cell transplantation (SSCT) could become a fertility restoration tool for childhood cancer survivors. However, since in mice, the colonization efficiency of transplanted spermatogonial stem cells (SSCs) is only 12%, the efficiency of the procedure needs to be improved before clinical implementation is possible. Co-transplantation of mesenchymal stem cells (MSCs) might increase colonization efficiency of SSCs by restoring the SSC niche after gonadotoxic treatment.
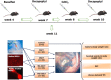
Methods: A mouse model for long-term infertility was developed and used to transplant SSCs (SSCT, n = 10), MSCs (MSCT, n = 10), a combination of SSCs and MSCs (MS-SSCT, n = 10), or a combination of SSCs and TGFß1-treated MSCs (MSi-SSCT, n = 10).
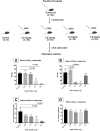
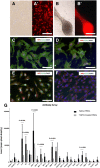
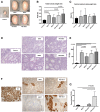
Results: The best model for transplantation was obtained after intraperitoneal injection of busulfan (40 mg/kg body weight) at 4 weeks followed by CdCl2 (2 mg/kg body weight) at 8 weeks of age and transplantation at 11 weeks of age. Three months after transplantation, spermatogenesis resumed with a significantly better tubular fertility index (TFI) in all transplanted groups compared to non-transplanted controls (P < 0.001). TFI after MSi-SSCT (83.3 ± 19.5%) was significantly higher compared to MS-SSCT (71.5 ± 21.7%, P = 0.036) but did not differ statistically compared to SSCT (78.2 ± 12.5%). In contrast, TFI after MSCT (50.2 ± 22.5%) was significantly lower compared to SSCT (P < 0.001). Interestingly, donor-derived TFI was found to be significantly improved after MSi-SSCT (18.8 ± 8.0%) compared to SSCT (1.9 ± 1.1%; P < 0.001), MSCT (0.0 ± 0.0%; P < 0.001), and MS-SSCT (3.4 ± 1.9%; P < 0.001). While analyses showed that both native and TGFß1-treated MSCs maintained characteristics of MSCs, the latter showed less migratory characteristics and was not detected in other organs.
Conclusion: Co-transplanting SSCs and TGFß1-treated MSCs significantly improves the recovery of endogenous SSCs and increases the homing efficiency of transplanted SSCs. This procedure could become an efficient method to treat infertility in a clinical setup, once the safety of the technique has been proven.
Keywords: Fertility restoration; Infertility; Mesenchymal stem cells; Spermatogonial stem cells; Transplantation.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed on the animals were in accordance with the ethical standards of the Federation for Laboratory Animal Science Associations guidelines and approved by the Institutional Animal Care and Use Committee of the Vrije Universiteit Brussel (VUB) Brussels, Belgium (14-216-2 and 16-216-3). Consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

