High-intensity versus low-intensity noninvasive positive pressure ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease (HAPPEN): study protocol for a multicenter randomized controlled trial
- PMID: 30463622
- PMCID: PMC6249746
- DOI: 10.1186/s13063-018-2991-y
High-intensity versus low-intensity noninvasive positive pressure ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease (HAPPEN): study protocol for a multicenter randomized controlled trial
Abstract
Background: Despite the positive outcomes of the use of noninvasive positive pressure ventilation (NPPV) in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD), NPPV fails in approximately 15% of patients with AECOPD, possibly because the inspiratory pressure delivered by conventional low-intensity NPPV is insufficient to improve ventilatory status for these patients. High-intensity NPPV, a novel form that delivers high inspiratory pressure, is believed to more efficiently augment alveolar ventilation than low-intensity NPPV, and it has been shown to improve ventilatory status more than low-intensity NPPV in stable AECOPD patients. Whether the application of high-intensity NPPV has therapeutic advantages over low-intensity NPPV in patients with AECOPD remains to be determined. The high-intensity versus low-intensity NPPV in patients with AECOPD (HAPPEN) study will examine whether high-intensity NPPV is more effective for correcting hypercapnia than low-intensity NPPV, hence reducing the need for intubation and improving survival.
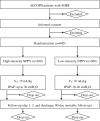
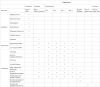
Methods/design: The HAPPEN study is a multicenter, two-arm, single-blind, prospective, randomized controlled trial. In total, 600 AECOPD patients with low to moderate hypercapnic respiratory failure will be included and randomized to receive high-intensity or low-intensity NPPV, with randomization stratified by study center. The primary endpoint is NPPV failure rate, defined as the need for endotracheal intubation and invasive ventilation. Secondary endpoints include the decrement of arterial carbon dioxide tension from baseline to 2 h after randomization, in-hospital and 28-day mortality, and 90-day survival. Patients will be followed up for 90 days after randomization.
Discussion: The HAPPEN study will be the first randomized controlled study to investigate whether high-intensity NPPV better corrects hypercapnia and reduces the need for intubation and mortality in AECOPD patients than low-intensity NPPV. The results will help critical care physicians decide the intensity of NPPV delivery to patients with AECOPD.
Trial registration: ClinicalTrials.gov, NCT02985918 . Registered on 7 December 2016.
Keywords: Chronic obstructive pulmonary disease; Endotracheal intubation; Exacerbation; High-intensity; Hypercapnia; Low-intensity; Mortality; Noninvasive positive pressure ventilation.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the ethics committee at each participating center. The protocol has reached version 6, in which there were no changes. Relevant amendments to the protocol will be submitted to the ethics committee for further approval, as warranted. Written informed consent to the study will be obtained from the patients, patients’ next of kin, or other surrogate decision makers where appropriate. A trained investigator is collecting the informed consent documents at each site.
Consent for publication
The written informed consent for the analysis and publication of anonymized data will be obtained from the patients, patients’ next of kin, or other surrogate decision makers, where appropriate.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effect of High-Intensity vs Low-Intensity Noninvasive Positive Pressure Ventilation on the Need for Endotracheal Intubation in Patients With an Acute Exacerbation of Chronic Obstructive Pulmonary Disease: The HAPPEN Randomized Clinical Trial.JAMA. 2024 Nov 26;332(20):1709-1722. doi: 10.1001/jama.2024.15815. JAMA. 2024. PMID: 39283649 Free PMC article. Clinical Trial.
-
Early use of noninvasive techniques for clearing respiratory secretions during noninvasive positive-pressure ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease and hypercapnic encephalopathy: A prospective cohort study.Medicine (Baltimore). 2017 Mar;96(12):e6371. doi: 10.1097/MD.0000000000006371. Medicine (Baltimore). 2017. PMID: 28328824 Free PMC article.
-
Home noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: a short-term prospective, multicenter, randomized, controlled trial.Int J Chron Obstruct Pulmon Dis. 2017 Apr 27;12:1279-1286. doi: 10.2147/COPD.S127540. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28490871 Free PMC article. Clinical Trial.
-
Efficacy of long-term noninvasive positive pressure ventilation in stable hypercapnic COPD patients with respiratory failure: a meta-analysis of randomized controlled trials.Int J Chron Obstruct Pulmon Dis. 2017 Oct 10;12:2977-2985. doi: 10.2147/COPD.S148422. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29066879 Free PMC article. Review.
-
Why High-Intensity NPPV is Favourable to Low-Intensity NPPV: Clinical and Physiological Reasons.COPD. 2017 Aug;14(4):389-395. doi: 10.1080/15412555.2017.1318843. Epub 2017 May 11. COPD. 2017. PMID: 28494170 Review.
Cited by
-
Non-Invasive Ventilatory Support In the Elderly.Curr Geriatr Rep. 2019 Sep;8(3):153-159. doi: 10.1007/s13670-019-00287-5. Epub 2019 Jun 13. Curr Geriatr Rep. 2019. PMID: 32509503 Free PMC article.
-
Effect of High-Intensity vs Low-Intensity Noninvasive Positive Pressure Ventilation on the Need for Endotracheal Intubation in Patients With an Acute Exacerbation of Chronic Obstructive Pulmonary Disease: The HAPPEN Randomized Clinical Trial.JAMA. 2024 Nov 26;332(20):1709-1722. doi: 10.1001/jama.2024.15815. JAMA. 2024. PMID: 39283649 Free PMC article. Clinical Trial.
-
Risk prediction models for non-invasive ventilation failure in patients with chronic obstructive pulmonary disease: A systematic review.Medicine (Baltimore). 2024 Dec 20;103(51):e40588. doi: 10.1097/MD.0000000000040588. Medicine (Baltimore). 2024. PMID: 39705475 Free PMC article.
References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. - DOI - PubMed
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. - DOI - PMC - PubMed
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

