Hypoxia potentiates monocyte-derived dendritic cells for release of tumor necrosis factor α via MAP3K8
- PMID: 30463908
- PMCID: PMC6294625
- DOI: 10.1042/BSR20182019
Hypoxia potentiates monocyte-derived dendritic cells for release of tumor necrosis factor α via MAP3K8
Abstract
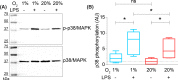
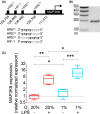
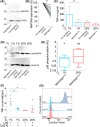
Dendritic cells (DCs) constantly sample peripheral tissues for antigens, which are subsequently ingested to derive peptides for presentation to T cells in lymph nodes. To do so, DCs have to traverse many different tissues with varying oxygen tensions. Additionally, DCs are often exposed to low oxygen tensions in tumors, where vascularization is lacking, as well as in inflammatory foci, where oxygen is rapidly consumed by inflammatory cells during the respiratory burst. DCs respond to oxygen levels to tailor immune responses to such low-oxygen environments. In the present study, we identified a mechanism of hypoxia-mediated potentiation of release of tumor necrosis factor α (TNF-α), a pro-inflammatory cytokine with important roles in both anti-cancer immunity and autoimmune disease. We show in human monocyte-derived DCs (moDCs) that this potentiation is controlled exclusively via the p38/mitogen-activated protein kinase (MAPK) pathway. We identified MAPK kinase kinase 8 (MAP3K8) as a target gene of hypoxia-induced factor (HIF), a transcription factor controlled by oxygen tension, upstream of the p38/MAPK pathway. Hypoxia increased expression of MAP3K8 concomitant with the potentiation of TNF-α secretion. This potentiation was no longer observed upon siRNA silencing of MAP3K8 or with a small molecule inhibitor of this kinase, and this also decreased p38/MAPK phosphorylation. However, expression of DC maturation markers CD83, CD86, and HLA-DR were not changed by hypoxia. Since DCs play an important role in controlling T-cell activation and differentiation, our results provide novel insight in understanding T-cell responses in inflammation, cancer, autoimmune disease and other diseases where hypoxia is involved.
Keywords: dendritic cells; hypoxia; inflammation; mitogen-activated protein kinases; tumour necrosis factors.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Activation of discoidin domain receptor 1 facilitates the maturation of human monocyte-derived dendritic cells through the TNF receptor associated factor 6/TGF-beta-activated protein kinase 1 binding protein 1 beta/p38 alpha mitogen-activated protein kinase signaling cascade.J Immunol. 2003 Oct 1;171(7):3520-32. doi: 10.4049/jimmunol.171.7.3520. J Immunol. 2003. Retraction in: J Immunol. 2010 Aug 1;185(3):1984. doi: 10.4049/jimmunol.1090057. PMID: 14500648 Retracted.
-
A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers.J Immunol. 2001 Mar 15;166(6):3837-45. doi: 10.4049/jimmunol.166.6.3837. J Immunol. 2001. PMID: 11238627
-
Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells.Int Immunol. 2004 Dec;16(12):1701-9. doi: 10.1093/intimm/dxh171. Epub 2004 Oct 11. Int Immunol. 2004. PMID: 15477228
-
Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration.Immunobiology. 2008;213(9-10):733-49. doi: 10.1016/j.imbio.2008.07.031. Epub 2008 Sep 21. Immunobiology. 2008. PMID: 18926289 Review.
-
Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement.J Dermatol Sci. 2006 Apr;42(1):1-11. doi: 10.1016/j.jdermsci.2005.11.004. Epub 2005 Dec 13. J Dermatol Sci. 2006. PMID: 16352421 Review.
Cited by
-
The Application of Drugs and Nano-Therapies Targeting Immune Cells in Hypoxic Inflammation.Int J Nanomedicine. 2024 Apr 9;19:3441-3459. doi: 10.2147/IJN.S456533. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38617798 Free PMC article. Review.
-
Oxygen in the tumor microenvironment: effects on dendritic cell function.Oncotarget. 2019 Jan 25;10(8):883-896. doi: 10.18632/oncotarget.26608. eCollection 2019 Jan 25. Oncotarget. 2019. PMID: 30783517 Free PMC article. Review.
-
MRTF may be the missing link in a multiscale mechanobiology approach toward macrophage dysfunction in space.Front Cell Dev Biol. 2022 Sep 12;10:997365. doi: 10.3389/fcell.2022.997365. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172272 Free PMC article.
-
Comparing the Blood Response to Hyperbaric Oxygen with High-Intensity Interval Training-A Crossover Study in Healthy Volunteers.Antioxidants (Basel). 2023 Nov 25;12(12):2043. doi: 10.3390/antiox12122043. Antioxidants (Basel). 2023. PMID: 38136163 Free PMC article.
-
Multimodal analysis for human ex vivo studies shows extensive molecular changes from delays in blood processing.iScience. 2021 Apr 15;24(5):102404. doi: 10.1016/j.isci.2021.102404. eCollection 2021 May 21. iScience. 2021. PMID: 34113805 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

