CRISPR/Cas9 and glycomics tools for Toxoplasma glycobiology
- PMID: 30463938
- PMCID: PMC6349120
- DOI: 10.1074/jbc.RA118.006072
CRISPR/Cas9 and glycomics tools for Toxoplasma glycobiology
Abstract
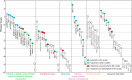
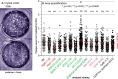
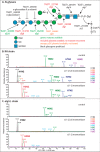
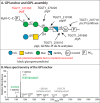
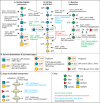
Infection with the protozoan parasite Toxoplasma gondii is a major health risk owing to birth defects, its chronic nature, ability to reactivate to cause blindness and encephalitis, and high prevalence in human populations. Unlike most eukaryotes, Toxoplasma propagates in intracellular parasitophorous vacuoles, but like nearly all other eukaryotes, Toxoplasma glycosylates many cellular proteins and lipids and assembles polysaccharides. Toxoplasma glycans resemble those of other eukaryotes, but species-specific variations have prohibited deeper investigations into their roles in parasite biology and virulence. The Toxoplasma genome encodes a suite of likely glycogenes expected to assemble N-glycans, O-glycans, a C-glycan, GPI-anchors, and polysaccharides, along with their precursors and membrane transporters. To investigate the roles of specific glycans in Toxoplasma, here we coupled genetic and glycomics approaches to map the connections between 67 glycogenes, their enzyme products, the glycans to which they contribute, and cellular functions. We applied a double-CRISPR/Cas9 strategy, in which two guide RNAs promote replacement of a candidate gene with a resistance gene; adapted MS-based glycomics workflows to test for effects on glycan formation; and infected fibroblast monolayers to assess cellular effects. By editing 17 glycogenes, we discovered novel Glc0-2-Man6-GlcNAc2-type N-glycans, a novel HexNAc-GalNAc-mucin-type O-glycan, and Tn-antigen; identified the glycosyltransferases for assembling novel nuclear O-Fuc-type and cell surface Glc-Fuc-type O-glycans; and showed that they are important for in vitro growth. The guide sequences, editing constructs, and mutant strains are freely available to researchers to investigate the roles of glycans in their favorite biological processes.
Keywords: CRISPR/Cas; Toxoplasma gondii; glycan; glycobiology; glycogene; glycomics; glycosyltransferase; mass spectrometry (MS); parasitology; protein glycosylation.
© 2019 Gas-Pascual et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

