Inhibition of ezrin causes PKCα-mediated internalization of erbb2/HER2 tyrosine kinase in breast cancer cells
- PMID: 30463939
- PMCID: PMC6341383
- DOI: 10.1074/jbc.RA118.004143
Inhibition of ezrin causes PKCα-mediated internalization of erbb2/HER2 tyrosine kinase in breast cancer cells
Abstract
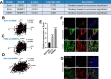
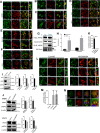
Unlike other ErbB family members, HER2 levels are maintained on the cell surface when the receptor is activated, allowing prolonged signaling and contributing to its transforming ability. Interactions between HER2, HSP90, PMCA2, and NHERF1 within specialized plasma membrane domains contribute to the membrane retention of HER2. We hypothesized that the scaffolding protein ezrin, which has been shown to interact with NHERF1, might also help stabilize the HER2-PMCA2-NHERF1 complex at the plasma membrane. Therefore, we examined ezrin expression and its relationship with HER2, NHERF1, and PMCA2 levels in murine and human breast cancers. We also used genetic knockdown and/or pharmacologic inhibition of ezrin, HSP90, NHERF1, PMCA2, and HER2 to examine the functional relationships between these factors and membrane retention of HER2. We found ezrin to be expressed at low levels at the apical surface of normal mammary epithelial cells, but its expression is up-regulated and correlates with HER2 expression in hyperplasia and tumors in murine mammary tumor virus-Neu mice, in human HER2-positive breast cancer cell lines, and in ductal carcinoma in situ and invasive breast cancers from human patients. In breast cancer cells, ezrin co-localizes and interacts with HER2, NHERF1, PMCA2, and HSP90 in specialized membrane domains, and inhibiting ezrin disrupts interactions between HER2, PMCA2, NHERF1, and HSP90, inhibiting HER2 signaling and causing PKCα-mediated internalization and degradation of HER2. Inhibition of ezrin synergizes with lapatinib in a PKCα-dependent fashion to inhibit proliferation and promote apoptosis in HER2-positive breast cancer cells. We conclude that ezrin stabilizes a multiprotein complex that maintains active HER2 at the cell surface.
Keywords: ErbB2/HER2; NHERF1; PDZ domain; PMCA2; breast cancer; ezrin; heat shock protein 90 (Hsp90); protein kinase C (PKC); scaffold protein.
© 2019 Jeong et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures






Similar articles
-
Erbin interacts with NHERF1 and Ezrin to stabilize a membrane ErbB2 signaling complex in HER2-positive breast cancer.Breast Cancer Res. 2025 May 19;27(1):85. doi: 10.1186/s13058-025-02025-6. Breast Cancer Res. 2025. PMID: 40390040 Free PMC article.
-
The scaffolding protein NHERF1 regulates the stability and activity of the tyrosine kinase HER2.J Biol Chem. 2017 Apr 21;292(16):6555-6568. doi: 10.1074/jbc.M116.770883. Epub 2017 Feb 24. J Biol Chem. 2017. PMID: 28235801 Free PMC article.
-
PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer.Proc Natl Acad Sci U S A. 2016 Jan 19;113(3):E282-90. doi: 10.1073/pnas.1516138113. Epub 2016 Jan 4. Proc Natl Acad Sci U S A. 2016. PMID: 26729871 Free PMC article.
-
Hormonally up-regulated neu-associated kinase: A novel target for breast cancer progression.Pharmacol Res. 2017 May;119:188-194. doi: 10.1016/j.phrs.2017.02.007. Epub 2017 Feb 9. Pharmacol Res. 2017. PMID: 28189783 Free PMC article. Review.
-
Imaging tumour heterogeneity of the consequences of a PKCα-substrate interaction in breast cancer patients.Biochem Soc Trans. 2014 Dec;42(6):1498-505. doi: 10.1042/BST20140165. Biochem Soc Trans. 2014. PMID: 25399560 Free PMC article. Review.
Cited by
-
A retrospective analysis of ezrin protein and mRNA expression in breast cancer: Ezrin expression is associated with patient survival and survival of patients with receptor-positive disease.Cancer Med. 2023 May;12(9):10908-10916. doi: 10.1002/cam4.5802. Epub 2023 Mar 20. Cancer Med. 2023. PMID: 36938826 Free PMC article.
-
Long Noncoding RNA EZR-AS1 Regulates the Proliferation, Migration, and Apoptosis of Human Venous Endothelial Cells via SMYD3.Biomed Res Int. 2020 May 22;2020:6840234. doi: 10.1155/2020/6840234. eCollection 2020. Biomed Res Int. 2020. PMID: 32596350 Free PMC article.
-
Translational Results of Zo-NAnTax: A Phase II Trial of Neoadjuvant Zoledronic Acid in HER2-Positive Breast Cancer.Int J Mol Sci. 2022 Dec 8;23(24):15515. doi: 10.3390/ijms232415515. Int J Mol Sci. 2022. PMID: 36555156 Free PMC article. Clinical Trial.
-
Advances and Future Perspectives of HER2 Mutations in Non-Small Lung Cancer (NSCLC), Especially in China.Cancer Control. 2025 Jan-Dec;32:10732748251347572. doi: 10.1177/10732748251347572. Epub 2025 Jun 5. Cancer Control. 2025. PMID: 40472040 Free PMC article. Review.
-
Cytoskeletal and Cytoskeleton-Associated Proteins: Key Regulators of Cancer Stem Cell Properties.Pharmaceuticals (Basel). 2022 Nov 8;15(11):1369. doi: 10.3390/ph15111369. Pharmaceuticals (Basel). 2022. PMID: 36355541 Free PMC article. Review.
References
-
- U.S. Cancer Statistics Working Group (2017) United States Cancer Statistics: 1999–2014. Incidence and Mortality Web-based Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, Atlanta, GA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

