TRPV1 pore turret dictates distinct DkTx and capsaicin gating
- PMID: 30463948
- PMCID: PMC6294906
- DOI: 10.1073/pnas.1809662115
TRPV1 pore turret dictates distinct DkTx and capsaicin gating
Abstract
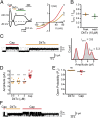
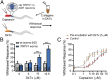
Many neurotoxins inflict pain by targeting receptors expressed on nociceptors, such as the polymodal cationic channel TRPV1. The tarantula double-knot toxin (DkTx) is a peptide with an atypical bivalent structure, providing it with the unique capability to lock TRPV1 in its open state and evoke an irreversible channel activation. Here, we describe a distinct gating mechanism of DkTx-evoked TRPV1 activation. Interestingly, DkTx evokes significantly smaller TRPV1 macroscopic currents than capsaicin, with a significantly lower unitary conductance. Accordingly, while capsaicin evokes aversive behaviors in TRPV1-transgenic Caenorhabditis elegans, DkTx fails to evoke such response at physiological concentrations. To determine the structural feature(s) responsible for this phenomenon, we engineered and evaluated a series of mutated toxins and TRPV1 channels. We found that elongating the DkTx linker, which connects its two knots, increases channel conductance compared with currents elicited by the native toxin. Importantly, deletion of the TRPV1 pore turret, a stretch of amino acids protruding out of the channel's outer pore region, is sufficient to produce both full conductance and aversive behaviors in response to DkTx. Interestingly, this deletion decreases the capsaicin-evoked channel activation. Taken together with structure modeling analysis, our results demonstrate that the TRPV1 pore turret restricts DkTx-mediated pore opening, probably through steric hindrance, limiting the current size and mitigating the evoked downstream physiological response. Overall, our findings reveal that DkTx and capsaicin elicit distinct TRPV1 gating mechanisms and subsequent pain responses. Our results also indicate that the TRPV1 pore turret regulates the mechanisms of channel gating and permeation.
Keywords: DkTx; TRPV1; capsaicin; pore turret; transgenic C. elegans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Structural insights into the mechanism of activation of the TRPV1 channel by a membrane-bound tarantula toxin.Elife. 2016 Feb 10;5:e11273. doi: 10.7554/eLife.11273. Elife. 2016. PMID: 26880553 Free PMC article.
-
High yield production and refolding of the double-knot toxin, an activator of TRPV1 channels.PLoS One. 2012;7(12):e51516. doi: 10.1371/journal.pone.0051516. Epub 2012 Dec 11. PLoS One. 2012. PMID: 23240036 Free PMC article.
-
Using C. elegans to Study the Effects of Toxins in Sensory Ion Channels In Vivo.Methods Mol Biol. 2020;2068:225-238. doi: 10.1007/978-1-4939-9845-6_12. Methods Mol Biol. 2020. PMID: 31576531
-
Understand spiciness: mechanism of TRPV1 channel activation by capsaicin.Protein Cell. 2017 Mar;8(3):169-177. doi: 10.1007/s13238-016-0353-7. Epub 2017 Jan 2. Protein Cell. 2017. PMID: 28044278 Free PMC article. Review.
-
Animal Toxins Providing Insights into TRPV1 Activation Mechanism.Toxins (Basel). 2017 Oct 16;9(10):326. doi: 10.3390/toxins9100326. Toxins (Basel). 2017. PMID: 29035314 Free PMC article. Review.
Cited by
-
Structural mechanisms of transient receptor potential ion channels.J Gen Physiol. 2020 Mar 2;152(3):e201811998. doi: 10.1085/jgp.201811998. J Gen Physiol. 2020. PMID: 31972006 Free PMC article. Review.
-
Pain-related toxins in scorpion and spider venoms: a face to face with ion channels.J Venom Anim Toxins Incl Trop Dis. 2021 Dec 6;27:e20210026. doi: 10.1590/1678-9199-JVATITD-2021-0026. eCollection 2021. J Venom Anim Toxins Incl Trop Dis. 2021. PMID: 34925480 Free PMC article. Review.
-
TRPV1: Structure, Endogenous Agonists, and Mechanisms.Int J Mol Sci. 2020 May 12;21(10):3421. doi: 10.3390/ijms21103421. Int J Mol Sci. 2020. PMID: 32408609 Free PMC article. Review.
-
Relation between flexibility and intrinsically disorder regions in thermosensitive TRP channels reveal allosteric effects.Eur Biophys J. 2024 Feb;53(1-2):77-90. doi: 10.1007/s00249-023-01682-9. Epub 2023 Sep 30. Eur Biophys J. 2024. PMID: 37777680
-
Permeant cations modulate pore dynamics and gating of TRPV1 ion channels.J Gen Physiol. 2024 Jan 1;156(1):e202313422. doi: 10.1085/jgp.202313422. Epub 2023 Dec 6. J Gen Physiol. 2024. PMID: 38055192 Free PMC article.
References
-
- Chacur M, et al. Snake venom components enhance pain upon subcutaneous injection: An initial examination of spinal cord mediators. Pain. 2004;111:65–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

