Defective Viral Genomes Alter How Sendai Virus Interacts with Cellular Trafficking Machinery, Leading to Heterogeneity in the Production of Viral Particles among Infected Cells
- PMID: 30463965
- PMCID: PMC6364009
- DOI: 10.1128/JVI.01579-18
Defective Viral Genomes Alter How Sendai Virus Interacts with Cellular Trafficking Machinery, Leading to Heterogeneity in the Production of Viral Particles among Infected Cells
Abstract
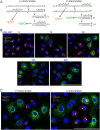
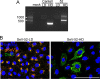
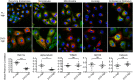
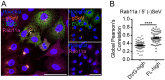
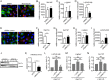
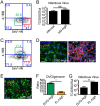
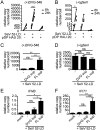
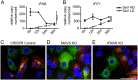
Defective viral genomes (DVGs) generated during RNA virus replication determine infection outcome by triggering innate immunity, diminishing virulence, and, in many cases, facilitating the establishment of persistent infections. Despite their critical role during virus-host interactions, the mechanisms regulating the production and propagation of DVGs are poorly understood. Visualization of viral genomes using RNA fluorescent in situ hybridization revealed a striking difference in the intracellular localization of DVGs and full-length viral genomes during infections with the paramyxovirus Sendai virus. In cells enriched in full-length virus, viral genomes clustered in a perinuclear region and associated with cellular trafficking machinery, including microtubules and the GTPase Rab11a. However, in cells enriched in DVGs, defective genomes distributed diffusely throughout the cytoplasm and failed to interact with this cellular machinery. Consequently, cells enriched in full-length genomes produced both DVG- and full-length-genome-containing viral particles, while DVG-high cells poorly produced viral particles yet strongly stimulated antiviral immunity. These findings reveal the selective production of both standard and DVG-containing particles by a subpopulation of infected cells that can be differentiated by the intracellular localization of DVGs. This study highlights the importance of considering this functional heterogeneity in analyses of virus-host interactions during infection.IMPORTANCE Defective viral genomes (DVGs) generated during Sendai virus infections accumulate in the cytoplasm of some infected cells and stimulate antiviral immunity and cell survival. DVGs are packaged and released as defective particles and have a significant impact on infection outcome. We show that the subpopulation of DVG-high cells poorly engages the virus packaging and budding machinery and do not effectively produce viral particles. In contrast, cells enriched in full-length genomes are the primary producers of both standard and defective viral particles during infection. This study demonstrates heterogeneity in the molecular interactions occurring within infected cells and highlights distinct functional roles for cells as either initiators of immunity or producers and perpetuators of viral particles depending on their content of viral genomes and their intracellular localization.
Keywords: defective interfering particles; defective viral genomes; infection heterogeneity; paramyxovirus; particle production.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Sun Y, Jain D, Koziol-White CJ, Genoyer E, Gilbert M, Tapia K, Panettieri RA, Hodinka RL, López CB. 2015. Immunostimulatory defective viral genomes from respiratory syncytial virus promote a strong innate antiviral response during infection in mice and humans. PLoS Pathog 11:e1005122. doi: 10.1371/journal.ppat.1005122. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

