Epidemiological Evidence for Lineage-Specific Differences in the Risk of Inapparent Chikungunya Virus Infection
- PMID: 30463967
- PMCID: PMC6364014
- DOI: 10.1128/JVI.01622-18
Epidemiological Evidence for Lineage-Specific Differences in the Risk of Inapparent Chikungunya Virus Infection
Abstract
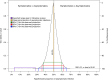
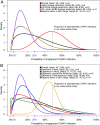
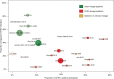
In late 2013, chikungunya virus (CHIKV) was introduced into the Americas, leading to widespread epidemics. A large epidemic caused by the Asian chikungunya virus (CHIKV) lineage occurred in Managua, Nicaragua, in 2015. Literature reviews commonly state that the proportion of inapparent CHIKV infections ranges from 3 to 28%. This study estimates the ratio of symptomatic to asymptomatic CHIKV infections and identifies risk factors of infection. In October to November 2015, 60 symptomatic CHIKV-infected children were enrolled as index cases and prospectively monitored, alongside 236 household contacts, in an index cluster study. Samples were collected upon enrollment and on day 14 or 35 and tested by real-time reverse transcription-PCR (rRT-PCR), IgM capture enzyme-linked immunosorbent assays (IgM-ELISAs), and inhibition ELISAs to detect pre- and postenrollment CHIKV infections. Of 236 household contacts, 55 (23%) had experienced previous or very recent infections, 41 (17%) had active infections at enrollment, and 21 (9%) experienced incident infections. Vehicle ownership (multivariable-adjusted risk ratio [aRR], 1.58) increased the risk of CHIKV infection, whereas ≥4 municipal trash collections/week (aRR, 0.38) and having externally piped water (aRR, 0.52) protected against CHIKV infection. Among 63 active and incident infections, 31 (49% [95% confidence interval {CI}, 36%, 62%]) were asymptomatic, yielding a ratio of symptomatic to asymptomatic infections of 1:0.97 (95% CI, 1:0.56, 1:1.60). Although our estimate is outside the 3% to 28% range reported previously, Bayesian and simulation analyses, informed by a systematic literature search, suggested that the proportion of inapparent CHIKV infections is lineage dependent and that more inapparent infections are associated with the Asian lineage than the East/Central/South African (ECSA) lineage. Overall, these data substantially improve knowledge regarding chikungunya epidemics.IMPORTANCE Chikungunya virus (CHIKV) is an understudied threat to human health. During the 2015 chikungunya epidemic in Managua, Nicaragua, we estimated the ratio of symptomatic to asymptomatic CHIKV infections, which is important for understanding transmission dynamics and the public health impact of CHIKV. This index cluster study identified and monitored persons at risk of infection, enabling capture of asymptomatic infections. We estimated that 31 (49%) of 63 at-risk participants had asymptomatic CHIKV infections, which is significantly outside the 3% to 28% range reported in literature reviews. However, recent seroprevalence studies, including two large pediatric cohort studies in the same setting, had also found percentages of inapparent infections outside the 3% to 28% range. Bayesian and simulation analyses, informed by a systematic literature search, revealed that the percentage of inapparent infections in epidemic settings varies by CHIKV phylogenetic lineage. Our study quantifies and provides the first epidemiological evidence that chikungunya epidemic characteristics are strongly influenced by CHIKV lineage.
Keywords: Bayesian analysis; S:A ratio; chikungunya virus; epidemics; index cluster study; lineage.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Centers for Disease Control and Prevention. 2015. Chikungunya virus: clinical evaluation and disease. Centers for Disease Control and Prevention, Atlanta, GA.
-
- Teo T-H, Her Z, Tan JJL, Lum F-M, Lee WWL, Chan Y-H, Ong R-Y, Kam Y-W, Leparc-Goffart I, Gallian P, Rénia L, de Lamballerie X, Ng LFP. 2015. Caribbean and La Réunion chikungunya virus isolates differ in their capacity to induce proinflammatory Th1 and NK cell responses and acute joint pathology. J Virol 89:7955–7969. doi: 10.1128/JVI.00909-15. - DOI - PMC - PubMed
-
- Langsjoen RM, Haller SL, Roy CJ, Vinet-Oliphant H, Bergren NA, Erasmus JH, Livengood JA, Powell TD, Weaver SC, Rossi SL. 2018. Chikungunya virus strains show lineage-specific variations in virulence and cross-protective ability in murine and nonhuman primate models. mBio 9:e02449-17. doi: 10.1128/mBio.02449-17. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

