RNA Helicase A Is an Important Host Factor Involved in Dengue Virus Replication
- PMID: 30463971
- PMCID: PMC6363994
- DOI: 10.1128/JVI.01306-18
RNA Helicase A Is an Important Host Factor Involved in Dengue Virus Replication
Abstract
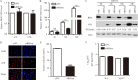
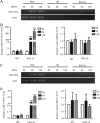
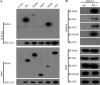
Dengue virus (DENV) utilizes host factors throughout its life cycle. In this study, we identified RNA helicase A (RHA), a member of the DEAD/H helicase family, as an important host factor of DENV. In response to DENV2 infection, nuclear RHA protein was partially redistributed into the cytoplasm. The short interfering RNA-mediated knockdown of RHA significantly reduced the amounts of infectious viral particles in various cells. The RHA knockdown reduced the multistep viral growth of DENV2 and Japanese encephalitis virus but not Zika virus. Further study showed that the absence of RHA resulted in a reduction of both viral RNA and protein levels, and the data obtained from the reporter replicon assay indicated that RHA does not directly promote viral protein synthesis. RHA bound to the DENV RNA and associated with three nonstructural proteins, including NS1, NS2B3, and NS4B. Further study showed that different domains of RHA mediated its interaction with these viral proteins. The expression of RHA or RHA-K417R mutant protein lacking ATPase/helicase activity in RHA-knockdown cells successfully restored DENV2 replication levels, suggesting that the helicase activity of RHA is dispensable for its proviral effect. Overall, our work reveals that RHA is an important factor of DENV and might serve as a target for antiviral agents.IMPORTANCE Dengue, caused by dengue virus, is a rapidly spreading disease, and currently there are no treatments available. Host factors involved in the viral replication of dengue virus are potential antiviral therapeutic targets. Although RHA has been shown to promote the multiplication of several viruses, such as HIV and adenovirus, its role in the flavivirus family, including dengue virus, Japanese encephalitis virus, and emerging Zika virus, remains elusive. The current study revealed that RHA relocalized into the cytoplasm upon DENV infection and associated with viral RNA and nonstructural proteins, implying that RHA was actively engaged in the viral life cycle. We further provide evidence that RHA promoted the viral yields of DENV2 independent of its helicase activity. These findings demonstrated that RHA is a new host factor required for DENV replication and might serve as a target for antiviral drugs.
Keywords: dengue virus; flavivirus; host factor; viral replication.
Copyright © 2019 American Society for Microbiology.
Figures




Similar articles
-
A Combined Genetic-Proteomic Approach Identifies Residues within Dengue Virus NS4B Critical for Interaction with NS3 and Viral Replication.J Virol. 2015 Jul;89(14):7170-86. doi: 10.1128/JVI.00867-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926641 Free PMC article.
-
NS5 from Dengue Virus Serotype 2 Can Adopt a Conformation Analogous to That of Its Zika Virus and Japanese Encephalitis Virus Homologues.J Virol. 2019 Dec 12;94(1):e01294-19. doi: 10.1128/JVI.01294-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597763 Free PMC article.
-
The Transactions of NS3 and NS5 in Flaviviral RNA Replication.Adv Exp Med Biol. 2018;1062:147-163. doi: 10.1007/978-981-10-8727-1_11. Adv Exp Med Biol. 2018. PMID: 29845531 Review.
-
The Dengue Virus Nonstructural Protein 1 (NS1) Interacts with the Putative Epigenetic Regulator DIDO1 to Promote Flavivirus Replication in Mosquito Cells.J Virol. 2022 Jun 22;96(12):e0070422. doi: 10.1128/jvi.00704-22. Epub 2022 Jun 2. J Virol. 2022. PMID: 35652656 Free PMC article.
-
Establishment and Application of Flavivirus Replicons.Adv Exp Med Biol. 2018;1062:165-173. doi: 10.1007/978-981-10-8727-1_12. Adv Exp Med Biol. 2018. PMID: 29845532 Review.
Cited by
-
Hydroxycarboxylic Acid Receptor 2 Is a Zika Virus Restriction Factor That Can Be Induced by Zika Virus Infection Through the IRE1-XBP1 Pathway.Front Cell Infect Microbiol. 2020 Jan 22;9:480. doi: 10.3389/fcimb.2019.00480. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 32039055 Free PMC article.
-
Inositol-Requiring Enzyme 1α Promotes Zika Virus Infection through Regulation of Stearoyl Coenzyme A Desaturase 1-Mediated Lipid Metabolism.J Virol. 2020 Nov 9;94(23):e01229-20. doi: 10.1128/JVI.01229-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32967957 Free PMC article.
-
Exploiting host kinases to combat dengue virus infection and disease.Antiviral Res. 2025 Sep;241:106172. doi: 10.1016/j.antiviral.2025.106172. Epub 2025 May 8. Antiviral Res. 2025. PMID: 40348023 Review.
-
Diacylglycerol O-acyltransferase 2, a Novel Target of Flavivirus NS2B3 Protease, Promotes Zika Virus Replication by Regulating Lipid Droplet Formation.Research (Wash D C). 2024 Oct 24;7:0511. doi: 10.34133/research.0511. eCollection 2024. Research (Wash D C). 2024. PMID: 39449854 Free PMC article.
-
Overview of host-directed antiviral targets for future research and drug development.Acta Pharm Sin B. 2025 Apr;15(4):1723-1751. doi: 10.1016/j.apsb.2025.03.011. Epub 2025 Mar 8. Acta Pharm Sin B. 2025. PMID: 40486850 Free PMC article. Review.
References
-
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castaneda-Orjuela CA, Chuang TW, Gibney KB, Memish ZA, Rafay A, Ukwaja KN, Yonemoto N, Murray CJL. 2016. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 16:712–723. doi:10.1016/S1473-3099(16)00026-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

