A Nanobody Targeting Viral Nonstructural Protein 9 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication
- PMID: 30463975
- PMCID: PMC6364029
- DOI: 10.1128/JVI.01888-18
A Nanobody Targeting Viral Nonstructural Protein 9 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication
Erratum in
-
Erratum for Wang et al., "A Nanobody Targeting Viral Nonstructural Protein 9 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication".J Virol. 2023 Jun 29;97(6):e0076923. doi: 10.1128/jvi.00769-23. Epub 2023 Jun 12. J Virol. 2023. PMID: 37306581 Free PMC article. No abstract available.
Abstract
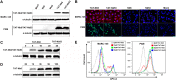
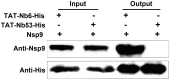
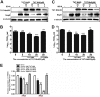
Porcine reproductive and respiratory syndrome (PRRS) is of great concern to the swine industry due to pandemic outbreaks of the disease, current ineffective vaccinations, and a lack of efficient antiviral strategies. In our previous study, a PRRSV Nsp9-specific nanobody, Nb6, was successfully isolated, and the intracellularly expressed Nb6 could dramatically inhibit PRRSV replication in MARC-145 cells. However, despite its small size, the application of Nb6 protein in infected cells is greatly limited, as the protein itself cannot enter the cells physically. In this study, a trans-activating transduction (TAT) peptide was fused with Nb6 to promote protein entry into cells. TAT-Nb6 was expressed as an inclusion body in Escherichia coli, and indirect enzyme-linked immunosorbent assays and pulldown assays showed that E. coli-expressed TAT-Nb6 maintained the binding ability to E. coli-expressed or PRRSV-encoded Nsp9. We demonstrated that TAT delivered Nb6 into MARC-145 cells and porcine alveolar macrophages (PAMs) in a dose- and time-dependent manner, and TAT-Nb6 efficiently inhibited the replication of several PRRSV genotype 2 strains as well as a genotype 1 strain. Using a yeast two-hybrid assay, Nb6 recognition sites were identified in the C-terminal part of Nsp9 and spanned two discontinuous regions (Nsp9aa454-551 and Nsp9aa599-646). Taken together, these results suggest that TAT-Nb6 can be developed as an antiviral drug for the inhibition of PRRSV replication and controlling PRRS disease.IMPORTANCE The pandemic outbreak of PRRS, which is caused by PRRSV, has greatly affected the swine industry. We still lack an efficient vaccine, and it is an immense challenge to control its infection. An intracellularly expressed Nsp9-specific nanobody, Nb6, has been shown to be able to inhibit PRRSV replication in MARC-145 cells. However, its application is limited, because Nb6 cannot physically enter cells. Here, we demonstrated that the cell-penetrating peptide TAT could deliver Nb6 into cultured cells. In addition, TAT-Nb6 fusion protein could suppress the replication of various PRRSV strains in MARC-145 cells and PAMs. These findings may provide a new approach for drug development to control PRRS.
Keywords: PRRSV; antiviral agents; nanobody.
Copyright © 2019 American Society for Microbiology.
Figures





Similar articles
-
Nanobody Nb6 fused with porcine IgG Fc as the delivering tag to inhibit porcine reproductive and respiratory syndrome virus replication in porcine alveolar macrophages.Vet Res. 2021 Feb 17;52(1):25. doi: 10.1186/s13567-020-00868-9. Vet Res. 2021. PMID: 33596995 Free PMC article.
-
An intracellularly expressed Nsp9-specific nanobody in MARC-145 cells inhibits porcine reproductive and respiratory syndrome virus replication.Vet Microbiol. 2015 Dec 31;181(3-4):252-60. doi: 10.1016/j.vetmic.2015.10.021. Epub 2015 Oct 26. Vet Microbiol. 2015. PMID: 26525739
-
A nanobody inhibiting porcine reproductive and respiratory syndrome virus replication via blocking self-interaction of viral nucleocapsid protein.J Virol. 2024 Jan 23;98(1):e0131923. doi: 10.1128/jvi.01319-23. Epub 2023 Dec 12. J Virol. 2024. PMID: 38084961 Free PMC article.
-
Mechanism of PRRSV infection and antiviral role of polyphenols.Virulence. 2024 Dec;15(1):2417707. doi: 10.1080/21505594.2024.2417707. Epub 2024 Oct 21. Virulence. 2024. PMID: 39432383 Free PMC article. Review.
-
Research Progress of Porcine Reproductive and Respiratory Syndrome Virus NSP2 Protein.Viruses. 2023 Nov 24;15(12):2310. doi: 10.3390/v15122310. Viruses. 2023. PMID: 38140551 Free PMC article. Review.
Cited by
-
A novel intracellularly expressed NS5B-specific nanobody suppresses bovine viral diarrhea virus replication.Vet Microbiol. 2020 Jan;240:108449. doi: 10.1016/j.vetmic.2019.108449. Epub 2019 Oct 12. Vet Microbiol. 2020. PMID: 31836380 Free PMC article.
-
Expression and immunogenicity of nsp10 protein of porcine epidemic diarrhea virus.Res Vet Sci. 2022 May;144:34-43. doi: 10.1016/j.rvsc.2021.12.024. Epub 2022 Jan 3. Res Vet Sci. 2022. PMID: 35038674 Free PMC article.
-
Nanobodies in the fight against infectious diseases: repurposing nature's tiny weapons.World J Microbiol Biotechnol. 2024 May 21;40(7):209. doi: 10.1007/s11274-024-03990-4. World J Microbiol Biotechnol. 2024. PMID: 38771414 Free PMC article. Review.
-
A review on camelid nanobodies with potential application in veterinary medicine.Vet Res Commun. 2024 Aug;48(4):2051-2068. doi: 10.1007/s11259-024-10432-x. Epub 2024 Jun 13. Vet Res Commun. 2024. PMID: 38869749 Review.
-
Viral replication organelles: the highly complex and programmed replication machinery.Front Microbiol. 2024 Jul 31;15:1450060. doi: 10.3389/fmicb.2024.1450060. eCollection 2024. Front Microbiol. 2024. PMID: 39144209 Free PMC article. Review.
References
-
- Liu H, Wang Y, Duan H, Zhang A, Liang C, Gao J, Zhang C, Huang B, Li Q, Li N, Xiao S, Zhou E-M. 2015. An intracellularly expressed Nsp9-specific nanobody in MARC-145 cells inhibits porcine reproductive and respiratory syndrome virus replication. Vet Microbiol 181:252–260. doi:10.1016/j.vetmic.2015.10.021. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

