Oncogenic Kaposi's Sarcoma-Associated Herpesvirus Upregulates Argininosuccinate Synthase 1, a Rate-Limiting Enzyme of the Citrulline-Nitric Oxide Cycle, To Activate the STAT3 Pathway and Promote Growth Transformation
- PMID: 30463977
- PMCID: PMC6364034
- DOI: 10.1128/JVI.01599-18
Oncogenic Kaposi's Sarcoma-Associated Herpesvirus Upregulates Argininosuccinate Synthase 1, a Rate-Limiting Enzyme of the Citrulline-Nitric Oxide Cycle, To Activate the STAT3 Pathway and Promote Growth Transformation
Abstract
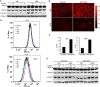
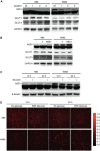
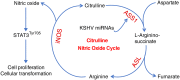
Cancer cells are required to rewire existing metabolic pathways to support their abnormal proliferation. We have previously shown that, unlike glucose-addicted cancers, Kaposi's sarcoma-associated herpesvirus (KSHV)-transformed cells depend on glutamine rather than glucose for energy production and amino acid and nucleotide syntheses. High-level consumption of glutamine is tightly regulated and often coupled with the citrulline-nitric oxide (NO) cycle. We have found that KSHV infection accelerates nitrogen efflux by upregulating the expression of argininosuccinate synthase 1 (ASS1), a key enzyme in the citrulline-NO cycle. KSHV utilizes multiple microRNAs to upregulate ASS1 expression. Depletion of either ASS1 or inducible nitric oxide synthase (iNOS) in KSHV-transformed cells suppresses growth proliferation, abolishes colony formation in soft agar, and decreases NO generation. Furthermore, by maintaining intracellular NO levels, ASS1 expression facilitates KSHV-mediated activation of the STAT3 pathway, which is critical for virus-induced transformation. These results illustrate a novel mechanism by which an oncogenic virus hijacks a key metabolic pathway to promote growth transformation and reveal a potential novel therapeutic target for KSHV-induced malignancies.IMPORTANCE We have previously shown that Kaposi's sarcoma-associated herpesvirus (KSHV)-transformed cells depend on glutamine rather than glucose for energy production and amino acid and nucleotide syntheses. In this study, we have further examined how the KSHV-reprogramed metabolic pathways are regulated and discovered that KSHV hijacks the citrulline-nitric oxide (NO) cycle to promote growth proliferation and transformation. Multiple KSHV-encoded microRNAs upregulate argininosuccinate synthase 1 (ASS1), a key enzyme in the citrulline-NO cycle. ASS1 is required for KSHV-induced proliferation, colony formation in soft agar, and NO generation of KSHV-transformed cells, which also depends on inducible nitric oxide synthase. By maintaining intracellular NO levels, ASS1 mediates KSHV activation of the STAT3 pathway, which is essential for KSHV-induced abnormal cell proliferation and transformation. These results illustrate a novel mechanism by which an oncogenic virus hijacks a key metabolic pathway to promote growth transformation and reveal a potential novel therapeutic target for KSHV-induced malignancies.
Keywords: ASS1; KSHV; Kaposi’s sarcoma; NO; STAT3; argininosuccinate synthase 1; citrulline-nitric oxide cycle; iNOS; inducible nitric oxide synthase; microRNA; nitric oxide.
Copyright © 2019 American Society for Microbiology.
Figures


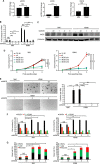
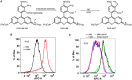
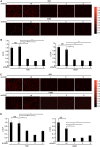
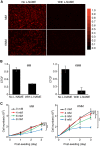
Similar articles
-
KSHV reprograms host RNA splicing via FAM50A to activate STAT3 and drive oncogenic cellular transformation.mBio. 2025 Jul 9;16(7):e0129325. doi: 10.1128/mbio.01293-25. Epub 2025 Jun 12. mBio. 2025. PMID: 40503897 Free PMC article.
-
Glycolysis, Glutaminolysis, and Fatty Acid Synthesis Are Required for Distinct Stages of Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication.J Virol. 2017 Apr 28;91(10):e02237-16. doi: 10.1128/JVI.02237-16. Print 2017 May 15. J Virol. 2017. PMID: 28275189 Free PMC article.
-
Exogenous arginine differentially regulates inflammatory cytokine and inducible nitric oxide synthase expression in macrophages.Immunohorizons. 2025 Jul 14;9(8):vlaf028. doi: 10.1093/immhor/vlaf028. Immunohorizons. 2025. PMID: 40694828 Free PMC article.
-
Citrullinemia Type I.2004 Jul 7 [updated 2022 Aug 18]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2004 Jul 7 [updated 2022 Aug 18]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301631 Free Books & Documents. Review.
-
RNA Modifications and Their Role in Regulating KSHV Replication and Pathogenic Mechanisms.J Med Virol. 2025 Jan;97(1):e70140. doi: 10.1002/jmv.70140. J Med Virol. 2025. PMID: 39740054 Free PMC article. Review.
Cited by
-
KSHV hijacks FoxO1 to promote cell proliferation and cellular transformation by antagonizing oxidative stress.J Med Virol. 2023 Mar;95(3):e28676. doi: 10.1002/jmv.28676. J Med Virol. 2023. PMID: 36929740 Free PMC article.
-
Targeting FoxO proteins induces lytic reactivation of KSHV for treating herpesviral primary effusion lymphoma.PLoS Pathog. 2023 Aug 18;19(8):e1011581. doi: 10.1371/journal.ppat.1011581. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37594999 Free PMC article.
-
Viral interleukin-6 encoded by an oncogenic virus promotes angiogenesis and cellular transformation by enhancing STAT3-mediated epigenetic silencing of caveolin 1.Oncogene. 2020 Jun;39(23):4603-4618. doi: 10.1038/s41388-020-1317-1. Epub 2020 May 11. Oncogene. 2020. PMID: 32393833 Free PMC article.
-
Hypoxic reactivation of Kaposi's sarcoma associated herpesvirus.Cell Insight. 2024 Sep 7;3(6):100200. doi: 10.1016/j.cellin.2024.100200. eCollection 2024 Dec. Cell Insight. 2024. PMID: 39391006 Free PMC article. Review.
-
The role of pioneering transcription factors, chromatin accessibility and epigenetic reprogramming in oncogenic viruses.Front Microbiol. 2025 Jun 16;16:1602497. doi: 10.3389/fmicb.2025.1602497. eCollection 2025. Front Microbiol. 2025. PMID: 40589575 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA197153/CA/NCI NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- R01 DE025465/DE/NIDCR NIH HHS/United States
- R01 CA177377/CA/NCI NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 CA132637/CA/NCI NIH HHS/United States
- R35 CA200422/CA/NCI NIH HHS/United States
- R01 HL110609/HL/NHLBI NIH HHS/United States
- R01 CA096512/CA/NCI NIH HHS/United States
- R01 CA124332/CA/NCI NIH HHS/United States
- R01 AI116585/AI/NIAID NIH HHS/United States
- R01 CA213275/CA/NCI NIH HHS/United States
- R01 DE023926/DE/NIDCR NIH HHS/United States
- P01 CA180779/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

