The Host DHX9 DExH-Box Helicase Is Recruited to Chikungunya Virus Replication Complexes for Optimal Genomic RNA Translation
- PMID: 30463980
- PMCID: PMC6364007
- DOI: 10.1128/JVI.01764-18
The Host DHX9 DExH-Box Helicase Is Recruited to Chikungunya Virus Replication Complexes for Optimal Genomic RNA Translation
Abstract
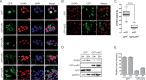
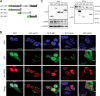
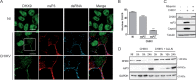
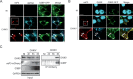
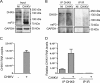
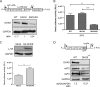
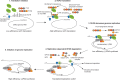
Beyond their role in cellular RNA metabolism, DExD/H-box RNA helicases are hijacked by various RNA viruses in order to assist replication of the viral genome. Here, we identify the DExH-box RNA helicase 9 (DHX9) as a binding partner of chikungunya virus (CHIKV) nsP3 mainly interacting with the C-terminal hypervariable domain. We show that during early CHIKV infection, DHX9 is recruited to the plasma membrane, where it associates with replication complexes. At a later stage of infection, DHX9 is, however, degraded through a proteasome-dependent mechanism. Using silencing experiments, we demonstrate that while DHX9 negatively controls viral RNA synthesis, it is also required for optimal mature nonstructural protein translation. Altogether, this study identifies DHX9 as a novel cofactor for CHIKV replication in human cells that differently regulates the various steps of CHIKV life cycle and may therefore mediate a switch in RNA usage from translation to replication during the earliest steps of CHIKV replication.IMPORTANCE The reemergence of chikungunya virus (CHIKV), an alphavirus that is transmitted to humans by Aedes mosquitoes, is a serious global health threat. In the absence of effective antiviral drugs, CHIKV infection has a significant impact on human health, with chronic arthritis being one of the most serious complications. The molecular understanding of host-virus interactions is a prerequisite to the development of targeted therapeutics capable to interrupt viral replication and transmission. Here, we identify the host cell DHX9 DExH-Box helicase as an essential cofactor for early CHIKV genome translation. We demonstrate that CHIKV nsP3 protein acts as a key factor for DHX9 recruitment to replication complexes. Finally, we establish that DHX9 behaves as a switch that regulates the progression of the viral cycle from translation to genome replication. This study might therefore have a significant impact on the development of antiviral strategies.
Keywords: DHX9; RNA helicase; chikungunya virus; nsP3; viral replication.
Copyright © 2019 American Society for Microbiology.
Figures



References
-
- Lemm JA, Rumenapf T, Strauss EG, Strauss JH, Rice CM. 1994. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J 13:2925–2934. doi: 10.1002/j.1460-2075.1994.tb06587.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

