Coordination of eukaryotic cilia and flagella
- PMID: 30464007
- PMCID: PMC6281475
- DOI: 10.1042/EBC20180029
Coordination of eukaryotic cilia and flagella
Abstract
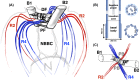
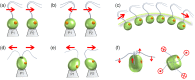
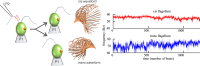
Propulsion by slender cellular appendages called cilia and flagella is an ancient means of locomotion. Unicellular organisms evolved myriad strategies to propel themselves in fluid environments, often involving significant differences in flagella number, localisation and modes of actuation. Remarkably, these appendages are highly conserved, occurring in many complex organisms such as humans, where they may be found generating physiological flows when attached to surfaces (e.g. airway epithelial cilia), or else conferring motility to male gametes (e.g. undulations of sperm flagella). Where multiple cilia arise, their movements are often observed to be highly coordinated. Here I review the two main mechanisms for motile cilia coordination, namely, intracellular and hydrodynamic, and discuss their relative importance in different ciliary systems.
Keywords: algae; centrioles/basal bodies; cilia; coordination; hydrodynamics; swimming.
© 2018 The Author(s).
Conflict of interest statement
The author declares that there are no competing interests associated with the manuscript.
Figures

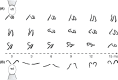
References
-
- Darwin C.R. (1875) The Movements and Habits of Climbing Plants, John Murray, London
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

