PGR5-Dependent Cyclic Electron Flow Protects Photosystem I under Fluctuating Light at Donor and Acceptor Sides
- PMID: 30464024
- PMCID: PMC6426425
- DOI: 10.1104/pp.18.01343
PGR5-Dependent Cyclic Electron Flow Protects Photosystem I under Fluctuating Light at Donor and Acceptor Sides
Abstract
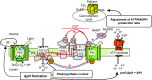
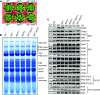
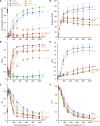
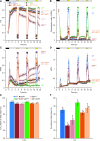
In response to a sudden increase in light intensity, plants must cope with absorbed excess photon energy to protect photosystems from photodamage. Under fluctuating light, PSI is severely photodamaged in the Arabidopsis (Arabidopsis thaliana) proton gradient regulation5 (pgr5) mutant defective in the main pathway of PSI cyclic electron transport (CET). Here, we aimed to determine how PSI is protected by two proposed regulatory roles of CET via transthylakoid ΔpH formation: (1) reservation of electron sink capacity by adjusting the ATP/NADPH production ratio (acceptor-side regulation) and (2) down-regulation of the cytochrome b 6 f complex activity called photosynthetic control for slowing down the electron flow toward PSI (donor-side regulation). We artificially enhanced donor- and acceptor-side regulation in the wild-type and pgr5 backgrounds by introducing the pgr1 mutation conferring the hypersensitivity of the cytochrome b 6 f complex to luminal acidification and moss Physcomitrella patens flavodiiron protein genes, respectively. Enhanced photosynthetic control partially alleviated PSI photodamage in the pgr5 mutant background but restricted linear electron transport under constant high light, suggesting that the strength of photosynthetic control should be optimized. Flavodiiron protein-dependent oxygen photoreduction formed a large electron sink and alleviated PSI photoinhibition, accompanied by the induction of photosynthetic control. Thus, donor-side regulation is essential for PSI photoprotection but acceptor-side regulation also is important to rapidly induce donor-side regulation. In angiosperms, PGR5-dependent CET is required for both functions.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures
Similar articles
-
Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis.Nat Plants. 2016 Feb 22;2:16012. doi: 10.1038/nplants.2016.12. Nat Plants. 2016. PMID: 27249347
-
Roles of the cyclic electron flow around PSI (CEF-PSI) and O₂-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana.Plant Cell Physiol. 2014 May;55(5):990-1004. doi: 10.1093/pcp/pcu033. Epub 2014 Feb 18. Plant Cell Physiol. 2014. PMID: 24553846
-
Flavodiiron Protein Substitutes for Cyclic Electron Flow without Competing CO2 Assimilation in Rice.Plant Physiol. 2018 Feb;176(2):1509-1518. doi: 10.1104/pp.17.01335. Epub 2017 Dec 14. Plant Physiol. 2018. PMID: 29242378 Free PMC article.
-
Contribution of Cyclic and Pseudo-cyclic Electron Transport to the Formation of Proton Motive Force in Chloroplasts.Mol Plant. 2017 Jan 9;10(1):20-29. doi: 10.1016/j.molp.2016.08.004. Epub 2016 Aug 26. Mol Plant. 2017. PMID: 27575692 Review.
-
Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis.Curr Opin Biotechnol. 2014 Apr;26:25-30. doi: 10.1016/j.copbio.2013.08.012. Epub 2013 Sep 21. Curr Opin Biotechnol. 2014. PMID: 24679254 Review.
Cited by
-
Photosynthetic electron transport rate and root dynamics of finger millet in response to Trichoderma harzianum.Plant Signal Behav. 2022 Dec 31;17(1):2146373. doi: 10.1080/15592324.2022.2146373. Plant Signal Behav. 2022. PMID: 36382615 Free PMC article.
-
Two dominant boreal conifers use contrasting mechanisms to reactivate photosynthesis in the spring.Nat Commun. 2020 Jan 8;11(1):128. doi: 10.1038/s41467-019-13954-0. Nat Commun. 2020. PMID: 31913273 Free PMC article.
-
Photosystem I Inhibition, Protection and Signalling: Knowns and Unknowns.Front Plant Sci. 2021 Dec 1;12:791124. doi: 10.3389/fpls.2021.791124. eCollection 2021. Front Plant Sci. 2021. PMID: 34925429 Free PMC article. Review.
-
Transcriptomic and Metabolomic Response to High Light in the Charophyte Alga Klebsormidium nitens.Front Plant Sci. 2022 May 6;13:855243. doi: 10.3389/fpls.2022.855243. eCollection 2022. Front Plant Sci. 2022. PMID: 35599877 Free PMC article.
-
Higher order photoprotection mutants reveal the importance of ΔpH-dependent photosynthesis-control in preventing light induced damage to both photosystem II and photosystem I.Sci Rep. 2020 Apr 21;10(1):6770. doi: 10.1038/s41598-020-62717-1. Sci Rep. 2020. PMID: 32317747 Free PMC article.
References
-
- Allen J. (2002) Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell 110: 273–276 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

