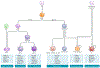
Fig. 1 ∣. Functionally specialized conventional and non-conventional dendritic cell subsets and related lineages.
The recent discovery of sets of particular transcription factors that define lineage ontogeny now allows for a clear distinction between monocytes and dendritic cells (DCs) as well as between different subsets of conventional DCs (cDCs) and non-cDCs,. cDCs derive from a common DC precursor (CDP), require the transcription factor FMS-like tyrosine kinase 3 ligand (FLT3L) for development and express, but do not require, the transcription factor ZBTB46 (REFS,). The genetic signature of cDCs from different tissues is similar but differs from that of plasmacytoid DCs (pDCs), monocytes and macrophages. As professional antigen-presenting cells, their primary function is to prime naive T cells. The BATF3-dependent and IRF8-dependent type 1 cDC (cDC1) subset expresses the chemokine XC receptor 1 (XCR1),, (in both mice and humans) and includes DCs that may express different surface markers such as CD8αα, DEC-205 or CD103. Shared expression of the surface receptor Langerin by both Langerhans cells (LCs) and dermal cDC1s initially caused confusion about the relative role of LCs in T cell priming, but newer models delineate the specific function of each population. The IRF4-dependent cDC2 subset can be identified by expression of surface markers CD11b, DC immunoreceptor 2 (DCIR2) (by staining with the antibody 33D1), CD301b (MGL2), CD4 or signal regulatory protein-α (SIRPα), depending on the tissue investigated,,,,. This subset includes the CD4+CD11b+ESAM+ DCs, which are dependent on the expression of NOTCH2 and the DNA-binding protein RBPJ, but also the double-negative (CD4−CD8−) DCs in the spleen, which are ESAMlow but CD11b+ (REFS,). Recent studies from other mouse tissues as well as human blood and lymph nodes (LNs) have similarly found two subsets of cDC2s,- (this division has not been depicted). Although some pDCs share a common developmental precursor with cDCs, they require distinct differentiation factors, express a unique pattern of surface markers and are functionally distinct from cDCs,. This includes being poor antigen-presenting cells for naive T cells (except under rare circumstances or potentially a unique subset) but robust producers of type I interferons,. One of the original DCs, LCs, has been re-classified with macrophages on the basis of ontogeny but performs many functions overlapping with cDCs. Both tissue macrophages and LCs derive from embryonic precursors and self-renew in situ (circular arrow indicates self-renewal in tissues). However, under inflammatory conditions, bone marrow-derived monocytes can differentiate to help repopulate both of these cells types. Tissue-resident macrophages maintain tissue homeostasis and a variety of other functions (reviewed previously). Macrophage populations also exist in LNs, but their roles are distinct from DCs; they are poor activators of naive T cells, even when presenting cognate antigen,,, but are potent at clearing apoptotic cells and promoting B cell activation,. Monocyte-derived DCs (MoDCs) include cells with a variety of functions, including TNF/iNOS-producing (TIP)-DCs as well as a difficult-to-define, heterogeneous group of cells collectively termed inflammatory DCs (iDCs). Like classical monocytes, MoDCs are dependent on the cytokine macrophage colony-stimulating factor (M-CSF), do not express the transcription factor ZBTB46 and depend on the chemokine receptor CC-chemokine receptor 2 (CCR2) for recruitment into inflammatory sites. They typically perform functions in the tissues such as antigen presentation to effector T cells, pathogen clearance and cytokine production. iDCs are induced during states of inflammation and have a mixed ontogeny and function that remain to be fully characterized,,. Some iDCs have, however, been shown to migrate to LNs and act more like cDCs, but these iDCs may in fact arrive in LNs through the blood via a CCR7-independent mechanism,. Select markers for each population are shown, with those in parentheses indicating heterogeneous or tissue-restricted expression. All populations express CD11c except monocytes. Below each population, the dominant (but not sole) function of each population is indicated. BDCA, blood DC antigen; CLEC9A, C-type lectin domain-containing 9A ; CLP, common lymphoid progenitor ; CX3CR1, CX3C-chemokine receptor 1; EPCAM, epithelial cell adhesion molecule; HSC, haematopoietic stem cell; MDP, macrophage DC progenitor ; SIGLECH, sialic acid-binding Ig-like lectin H. *Human-specific marker.