Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release
- PMID: 30464343
- PMCID: PMC6331005
- DOI: 10.1038/s41586-018-0735-5
Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release
Abstract
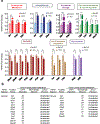
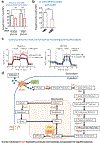
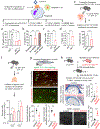
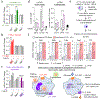
Development and routine tissue homeostasis require a high turnover of apoptotic cells. These cells are removed by professional and non-professional phagocytes via efferocytosis1. How a phagocyte maintains its homeostasis while coordinating corpse uptake, processing ingested materials and secreting anti-inflammatory mediators is incompletely understood1,2. Here, using RNA sequencing to characterize the transcriptional program of phagocytes actively engulfing apoptotic cells, we identify a genetic signature involving 33 members of the solute carrier (SLC) family of membrane transport proteins, in which expression is specifically modulated during efferocytosis, but not during antibody-mediated phagocytosis. We assessed the functional relevance of these SLCs in efferocytic phagocytes and observed a robust induction of an aerobic glycolysis program, initiated by SLC2A1-mediated glucose uptake, with concurrent suppression of the oxidative phosphorylation program. The different steps of phagocytosis2-that is, 'smell' ('find-me' signals or sensing factors released by apoptotic cells), 'taste' (phagocyte-apoptotic cell contact) and 'ingestion' (corpse internalization)-activated distinct and overlapping sets of genes, including several SLC genes, to promote glycolysis. SLC16A1 was upregulated after corpse uptake, increasing the release of lactate, a natural by-product of aerobic glycolysis3. Whereas glycolysis within phagocytes contributed to actin polymerization and the continued uptake of corpses, lactate released via SLC16A1 promoted the establishment of an anti-inflammatory tissue environment. Collectively, these data reveal a SLC program that is activated during efferocytosis, identify a previously unknown reliance on aerobic glycolysis during apoptotic cell uptake and show that glycolytic by-products of efferocytosis can influence surrounding cells.
Conflict of interest statement
The authors declare no competing financial interests. Correspondence and request for materials should be addressed to:
Figures









Comment in
-
Transporters help clear cell corpses.Nat Rev Immunol. 2019 Jan;19(1):5. doi: 10.1038/s41577-018-0100-9. Nat Rev Immunol. 2019. PMID: 30523286 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL120840/HL/NHLBI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 DK105550/DK/NIDDK NIH HHS/United States
- F31 DK108553/DK/NIDDK NIH HHS/United States
- R35 GM122542/GM/NIGMS NIH HHS/United States
- P30 DK056350/DK/NIDDK NIH HHS/United States
- R01 HL136664/HL/NHLBI NIH HHS/United States
- R01 CA217987/CA/NCI NIH HHS/United States
- R01 GM064709/GM/NIGMS NIH HHS/United States
- T32 CA009109/CA/NCI NIH HHS/United States
- T32 HL007284/HL/NHLBI NIH HHS/United States
- R01 NS072212/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

