Hydralazine HCl rapidly disintegrating sublingual tablets: simple dosage form of higher bioavailability and enhanced clinical efficacy for potential rapid control on hypertensive preeclampsia
- PMID: 30464406
- PMCID: PMC6225918
- DOI: 10.2147/DDDT.S173326
Hydralazine HCl rapidly disintegrating sublingual tablets: simple dosage form of higher bioavailability and enhanced clinical efficacy for potential rapid control on hypertensive preeclampsia
Abstract
Background: Hypertensive disorders are the most common complication in pregnancy which can even lead to maternal mortality. Hydralazine hydrochloride (HHC), a direct-acting vasodilator, is intravenously used as the first-line therapy in controlling hypertension in pregnancy (preeclampsia). It suffers poor oral bioavailability (26%-50%) due to first-pass metabolism.
Objective: This work aims for the preparation of HHC rapidly disintegrating sublingual tablets of higher absorption rate, short onset of action, and higher bioavailability for rapid control on blood pressure (BP) in hypertensive emergencies especially preeclampsia.
Methods: HHC sublingual tablet mixtures were prepared using starch sodium glycolate and Pharmaburst as super disintegrants at three different levels by direct compression and were subjected to full in vitro evaluation; the drug bioavailability from the optimized sublingual tablet formula was assessed in comparison to conventional oral tablets in rabbits, and the clinical efficacy on controlling BP in induced preeclampsia like mouse model was also studied.
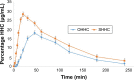
Results: The results indicated compatibility of the prepared tablet mixtures, good flow, and acceptable mechanical strength. Sublingual tablet formula containing Pharmaburst (7%) that showed fastest disintegration (21 seconds) and 100% drug release within 5 minutes was selected for further bioavailability and pharmacodynamic studies. The drug bioavailability was significantly increased with C max = 28.2767±4.61 µg/mL, AUC(0-α) = 52.85±3.18 µg.h/mL, and T max = 0.33±0.011 hour in comparison to 18.0633±23.2 µg/mL, 33.18±5.18 µg⋅h/mL, and 0.75±0.025 hour for conventional oral tablets. Results of pharmacodynamic studies proved significant rapid control on both systolic and diastolic BP to normal values within only 30 minutes without any significant difference from intravenous data.
Conclusion: These results confirm the suitability of the prepared HHC sublingual tablets for use in rapid control on hypertensive crisis especially in pregnant women as an alternate to parenteral administration.
Keywords: absorption from buccal cavity; hypertension; l-NAME; pre-eclampsia like mouse model; preeclampsia; sublingual tablets.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




References
-
- Weber MA, Schiffrin EL, White WB. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens. 2014;32(1):3–15. - PubMed
-
- James PA, Oparil S, Carter BL. Evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311(5):507–520. - PubMed
-
- Eckel RH, Jakicic JM, Ard JD, et al. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;2014(129):S76–S99. - PubMed
-
- Lewis G, Drife J, Die WM. Maternal Deaths in the United Kingdom. London: RCOG Press; 2001. The Fifth Report of the Confidential Enquiries into; pp. 1997–1999.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

