Analysis of a pharmacist-led adverse drug event management model for pharmacovigilance in an academic medical center hospital in China
- PMID: 30464487
- PMCID: PMC6214595
- DOI: 10.2147/TCRM.S178297
Analysis of a pharmacist-led adverse drug event management model for pharmacovigilance in an academic medical center hospital in China
Abstract
Background: Spontaneous reporting of adverse drug events (ADEs) has long been the cornerstone of pharmacovigilance. Medical institutions in China have been a major source of ADE case reports, but the proportion of reports from tertiary hospitals is low due to the serious underreporting of case reports. The same problem existed in the Second Affiliated Hospital of Zhejiang University School of Medicine (SAHZU).
Objective: In order to increase the number of ADE reports and promote hospital pharmacovigilance, SAHZU's clinical pharmacists established a pharmacist-led ADE management model. The aim of this paper is to introduce this management model and explore the advantages and disadvantages of the model.
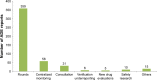
Methods: Pharmacist-led ADE management model was gradually formed from 2015 to 2017 in the SAHZU. This "pharmacist-led" model is reflected not only in the fact that clinical pharmacists are the main reporters of SAHZU's ADEs but also in that they are the main groups to analyze and manage ADE and drug errors. The sources of ADEs reported by clinical pharmacists mainly include pharmacy rounds, ADE-related pharmacist consultations, centralized monitoring, ADE warning signal analysis, newly introduced drug evaluations, and drug safety research.
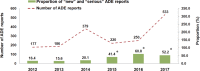
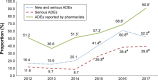
Results: A total of 533 ADEs were reported by SAHZU to China's spontaneous reporting system (SRS) in 2017, while the data in 2012 was 177, with an increase by 201%. In 2012, the proportion of "new" and "serious" reports was 16.4%. The proportions during the period from 2015 to 2017 were 41.4%, 60.8%, and 52.2%, respectively, which were statistically significant compared with the proportion in 2012. The proportion of ADEs reported by clinical pharmacists during the period from 2014 to 2017 were 51.5%, 57.3%, 68.8%, and 90.8%, respectively, which were statistically significant compared with the proportion in 2013 (P<0.05). There was a correlation between the proportions of severe ADEs and the proportion of ADEs reported by clinical pharmacists (r=0.873, P=0.023). Four hundred eighty four ADE cases reported by clinical pharmacists to China's SRS in 2017 were mainly found in rounds of clinical pharmacists (74.17% [359/484]).
Conclusion: The pharmacist-led pharmacovigilance working model significantly increased the quantity and quality of ADE reporting in SAHZU and promoted pharmacovigilance. This model is worth developing in Chinese tertiary hospitals and the following hospitals, where the physicians working there spend little time and energy on ADE reporting or the cost of physicians is high, while the clinical pharmacist team has strong professional skills.
Keywords: active surveillance; clinical pharmacist; new adverse drug events; serious adverse drug events; tertiary hospitals.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- World Health Organization The Importance of Pharmacovigilance – Safety Monitoring of Medicinal Products. 2002. [Accessed June 14, 2018]. Available from: http://apps.who.int/medicinedocs/en/d/Js4893e/
-
- Li X, Li H, Deng J, et al. Active pharmacovigilance in China: recent development and future perspectives. Eur J Clin Pharmacol. 2018;74(7):863–871. - PubMed
-
- Dong D, Liu CL. The Revelation of Study on Drug Manufacture Post Marketing Surveillance System in the United States. Chinese Journal of pharmacovigilance. 2013;10(8):456–459.
-
- Food and Drug Administration FAERS Reporting by Healthcare Providers and Consumers by Year. 2015. [Accessed June 14, 2018]. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveil....
LinkOut - more resources
Full Text Sources

