ADAMTS8 targets ERK to suppress cell proliferation, invasion, and metastasis of hepatocellular carcinoma
- PMID: 30464505
- PMCID: PMC6214590
- DOI: 10.2147/OTT.S173360
ADAMTS8 targets ERK to suppress cell proliferation, invasion, and metastasis of hepatocellular carcinoma
Abstract
Introduction: Hepatocellular carcinoma (HCC) is one of the most common malignant tumors of the digestive system. A disintegrin and metallopeptidase with thrombospondin motif (ADAMTS) has been identified as a secreted metalloproteinase that participates in the inhibition of tumor cell growth and invasion. The aims of the present study were to investigate the clinical significance of ADAMTS8 in patients with HCC and to determine the effect of ADAMTS8 on HCC cell biological activity in vitro and in vivo.
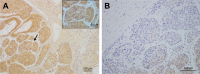
Methods: The tumor tissues and matched adjacent non-tumor tissues were collected from 61 patients with HCC, and ADAMTS8 expression was detected with immunohistochemistry. Flow cytometry and MTT assays were used to assess cell apoptosis and cell viability, respectively, and ERK, p-ERK, Stat3, p-Stat3, Akt, and p-Akt protein expressions were measured by Western blot.
Results: The results showed that ADAMTS8 expression was significantly lower in HCC tissues than that in adjacent non-tumor tissues. Moreover, ADAMTS8 expression was inversely associated with clinical stages and metastasis in patients with HCC. Furthermore, we found that transfection with exogenous ADAMTS8 inhibited proliferation and migration and induced apoptosis in HepG2 cells. In the in vivo study, tumor growth of upregulated HepG2 cells in nude mice was significantly slower. Moreover, decreased ERK activity was detected after transfection with ADAMTS8.
Conclusion: These results indicate that low ADAMTS8 expression is a predictor of a poor prognosis in patients with HCC and that ADAMTS8 plays an important role in regulating HCC growth, invasion, and apoptosis by modulating the ERK signaling pathway. ADAMTS8 maybe a new target in HCC treatment.
Keywords: ADAMT8; apoptosis; hepatocellular carcinoma; invasion and metastasis; signaling pathway.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- Tang BL. ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol. 2001;33(1):33–44. - PubMed
-
- Rocks N, Paulissen G, El Hour M, et al. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie. 2008;90(2):369–379. - PubMed
-
- Tortorella MD, Burn TC, Pratta MA, et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284(5420):1664–1666. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

