A meta-analysis of nivolumab for the treatment of advanced non-small-cell lung cancer
- PMID: 30464517
- PMCID: PMC6217212
- DOI: 10.2147/OTT.S171072
A meta-analysis of nivolumab for the treatment of advanced non-small-cell lung cancer
Abstract
Background: Non-small-cell lung cancer (NSCLC) is often associated with rapid progression following standard chemotherapy. Nivolumab, an inhibitor of PD-1/PD-L1, is reported to have potential efficacy for the treatment NSCLC.
Objective: The purpose of this meta-analysis was to systematically evaluate the efficacy and safety of nivolumab in patients with advanced NSCLC.
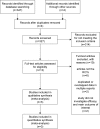
Methods: Online electronic databases were searched in June 2017, including: PubMed, Embase, and the Cochrane Library. Randomized controlled trials were included that compared nivolumab to chemotherapy in NSCLC patients with regard to oncological outcome profiles. Review Manager Version 5.3 software was used.
Results: Three studies were included in this analysis, comprising 1,395 patients with NSCLC, of whom 698 received nivolumab and 697 received chemotherapy without nivolumab. The pooled hazard ratios for overall survival (OS) and prolonged progression-free survival (PFS) were 0.77 (95% CI: 0.57-1.03; P=0.08) and 0.88 (95% CI: 0.64-1.20; P=0.41), respectively. The pooled odds ratio for overall response rate was 1.40 (95% CI: 0.66-2.96; P=0.39), indicating that no benefit with nivolumab was found for OS, PFS, or overall response rate. However, the odds ratio for treatment-related adverse events, grades 3 or 4, between the patients who received nivolumab and chemotherapy was 0.13 (95% CI: 0.09-0.17; P<0.00001). For patients with a PD-L1 expression level of 5% or more, no difference was observed in PFS (95% CI: 0.70-1.00; P=0.05) and OS benefit (95% CI: 0.34-1.15; P=0.13) between the groups.
Conclusion: These data demonstrate no clinical survival benefit with nivolumab for NSCLC patients, even in a subpopulation of patients with levels of PD-L1>5%. However, nivolumab had a more favorable safety profile than chemotherapy. Future investigations are needed to determine whether the efficacy of nivolumab can be improved.
Keywords: PD-1; meta-analysis; nivolumab; non-small-cell lung cancer.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
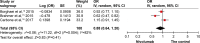





Similar articles
-
The relationship between blood-based tumor mutation burden level and efficacy of PD-1/PD-L1 inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Nov 13;21(1):1220. doi: 10.1186/s12885-021-08924-z. BMC Cancer. 2021. PMID: 34774004 Free PMC article.
-
Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: A meta-analysis of randomized clinical trials.Medicine (Baltimore). 2016 Aug;95(35):e4611. doi: 10.1097/MD.0000000000004611. Medicine (Baltimore). 2016. PMID: 27583876 Free PMC article. Review.
-
Comparative efficacy and safety of PD-1/PD-L1 Inhibitors versus platinum-based chemotherapy for the first-line treatment of advanced non-small cell lung cancer: a meta analysis of randomized controlled trials.Pharmacol Res. 2020 Oct;160:105194. doi: 10.1016/j.phrs.2020.105194. Epub 2020 Sep 13. Pharmacol Res. 2020. PMID: 32937178
-
The efficacy and safety of nivolumab in previously treated advanced non-small-cell lung cancer: a meta-analysis of prospective clinical trials.Onco Targets Ther. 2016 Sep 23;9:5867-5874. doi: 10.2147/OTT.S115262. eCollection 2016. Onco Targets Ther. 2016. PMID: 27713640 Free PMC article.
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
Cited by
-
Preclinical Characterization of GLS-010 (Zimberelimab), a Novel Fully Human Anti-PD-1 Therapeutic Monoclonal Antibody for Cancer.Front Oncol. 2021 Sep 15;11:736955. doi: 10.3389/fonc.2021.736955. eCollection 2021. Front Oncol. 2021. PMID: 34604074 Free PMC article.
-
The overall safety evaluation of programmed cell death/programmed cell death ligand 1 (PD-1/PD-L1) treatment for lung cancer patients: An updated systematic review and meta-analysis.Medicine (Baltimore). 2019 Jul;98(30):e16439. doi: 10.1097/MD.0000000000016439. Medicine (Baltimore). 2019. PMID: 31348245 Free PMC article.
-
Effectiveness and Safety of Immune Checkpoint Inhibitors for Patients with Advanced Non Small-Cell Lung Cancer in Real-World: Review and Meta-Analysis.Cancers (Basel). 2021 Mar 19;13(6):1388. doi: 10.3390/cancers13061388. Cancers (Basel). 2021. PMID: 33808533 Free PMC article. Review.
-
Efficacy and safety of PD-1/PD-L1 inhibitors plus nab-paclitaxel for patients with non-small cell lung cancer who have progressed after platinum-based chemotherapy.Ther Adv Med Oncol. 2020 Jul 6;12:1758835920936882. doi: 10.1177/1758835920936882. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 32670420 Free PMC article.
References
-
- Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32(4):669–692. - PubMed
-
- Chang S, Dai M, Ren JS, Chen YH, Guo LW. Estimates and prediction on incidence, mortality and prevalence of lung cancer in China in 2008. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33(4):391–394. - PubMed
-
- Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185–197. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

