Noninvasive screening identifies patients at risk for spontaneous bacterial peritonitis caused by multidrug-resistant organisms
- PMID: 30464547
- PMCID: PMC6223386
- DOI: 10.2147/IDR.S172587
Noninvasive screening identifies patients at risk for spontaneous bacterial peritonitis caused by multidrug-resistant organisms
Abstract
Background and aims: Spontaneous bacterial peritonitis (SBP) is a severe complication of decompensated cirrhosis. The prevalence of multidrug-resistant organisms (MDROs) in patients with cirrhosis is increasing. Identification of patients at risk for SBP due to MDROs (ie, SBP with the evidence of MDROs or Stenotrophomonas maltophilia in ascitic culture, MDRO-SBP) is crucial to the early adaptation of antibiotic treatment in such patients. We therefore investigated whether MDROs found in ascitic cultures can also be found in specimens determined by noninvasive screening procedures.
Patients and methods: This retrospective study was conducted at the liver center of the University Hospital Frankfurt, Germany. Between 2011 and 2016, patients with cirrhosis were included upon diagnosis of SBP and sample collection of aerobic/anaerobic ascitic cultures. Furthermore, the performance of at least one complete MDRO screening was mandatory for study inclusion.
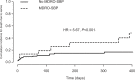
Results: Of 133 patients diagnosed with SBP, 75 (56.4%) had culture-positive SBP and 22 (16.5%) had MDRO-SBP. Multidrug-resistant Escherichia coli (10/22; 45.5%) and vancomycin-resistant enterococci (7/22; 36.4%) resembled the major causatives of MDRO-SBP. Rectal swabs identified MDROs in 17 of 22 patients (77.3%) who developed MDRO-SBP with a time-dependent sensitivity of 77% and 87% after 30 and 90 days upon testing, while negative predictive value was 83% and 76%, respectively. The majority of patients were included from intensive care unit or intermediate care unit.
Conclusion: MDRO screening may serve as a noninvasive diagnostic tool to identify patients at risk for MDRO-SBP. Patients with decompensated cirrhosis should be screened for MDROs from the first day of inpatient treatment onward.
Keywords: antibiotic therapy; ascites; liver cirrhosis; multidrug resistance; screening routine.
Conflict of interest statement
Disclosure SZ has provided consultancy for Abbvie, BMS, Gilead, Intercept, Janssen and Merck (all outside the scope of this study). OW has provided consultancy for Amgen, Bayer, BMS, Celgene, Eisai, Merck, Novartis, Roche, Servier and Shire (all outside the scope of this study). VAJK was partially supported by a grant from the Deutsche Forschungsgemein-schaft (DFG, research unit 2251). The authors report no other conflicts of interest in this work.
Figures
References
-
- Fernández J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55(5):1551–1561. - PubMed
-
- Garcia-Tsao G. Current Management of the Complications of Cirrhosis and Portal Hypertension: Variceal Hemorrhage, Ascites, and Spontaneous Bacterial Peritonitis. Dig Dis. 2016;34(4):382–386. - PubMed
-
- Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4):1246–1256. - PubMed
LinkOut - more resources
Full Text Sources

