Riluzole induces LTD of spinal nociceptive signaling via postsynaptic GluR2 receptors
- PMID: 30464577
- PMCID: PMC6209077
- DOI: 10.2147/JPR.S169686
Riluzole induces LTD of spinal nociceptive signaling via postsynaptic GluR2 receptors
Abstract
Purpose: Riluzole - a major therapeutic medicine for patients with amyotrophic lateral sclerosis - reportedly has anti-nociceptive and anti-allodynic efficacies in neuropathic pain models. However, little is known about its effect on neurotransmission in the spinal superficial dorsal horn (SDH). The present study aims to investigate the effects of riluzole on the synaptic transmission of SDH nociceptive pathways in both physiological and pathological conditions.
Materials and methods: Spinal nerve ligation was used to produce a neuropathic pain model. Mechanical allodynia behavior was assessed with Von Frey filaments. Riluzole's effects on nociceptive synaptic transmission under both physiological and pathological conditions were examined by patch-clamp recordings in rat SDH neurons.
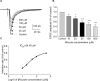
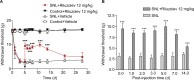
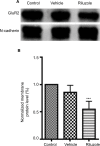
Results: The principal findings of the present study are three-fold. First, we affirm that riluzole has a remarkable long-lasting analgesic effect on both in vitro and in vivo pathological pain models. Second, the prolonged inhibitory effects of riluzole on spinal nociceptive signaling are mediated by both presynaptic and postsynaptic mechanisms. Finally, endocytosis of post-synaptic GluR2 contributes to the riluzole-induced long-term depression (LTD) of the spinal nociceptive pathway.
Conclusion: The present study finds that riluzole induces LTD of nociceptive signaling in the SDH and produces long-lasting anti-allodynia effects in nerve injury-induced neuropathic pain conditions via postsynaptic AMPA receptors associated with the endocytosis of GluR2.
Keywords: AMPA receptor endocytosis; LTD; SSDH; long-term depression; neuropathic pain; riluzole; superficial dorsal horn.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




References
-
- Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978;177(3):417–434. - PubMed
-
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186(2):133–150. - PubMed
-
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234(4774):358–361. - PubMed
-
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189–5194. - PMC - PubMed
-
- Irifune M, Kikuchi N, Saida T, et al. Riluzole, a glutamate release inhibitor, induces loss of righting reflex, antinociception, and immobility in response to noxious stimulation in mice. Anesth Analg. 2007;104(6):1415–1421. - PubMed
LinkOut - more resources
Full Text Sources

