Response to duloxetine in patients with knee pain due to osteoarthritis: an exploratory post hoc analysis of a Japanese Phase III randomized study
- PMID: 30464579
- PMCID: PMC6209071
- DOI: 10.2147/JPR.S176036
Response to duloxetine in patients with knee pain due to osteoarthritis: an exploratory post hoc analysis of a Japanese Phase III randomized study
Abstract
Purpose: To assess whether patients with knee osteoarthritis pain who have early pain reduction or treatment-related adverse events of special interest (TR-AESIs; constipation, decreased appetite, malaise, nausea, somnolence, thirst) with duloxetine treatment are more likely to have later improvements in pain and quality of life (QOL) relative to placebo than patients without these early indicators.
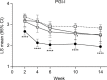
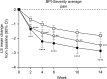
Patients and methods: This was a post hoc analysis of 14-week randomized trial of Japanese patients with knee osteoarthritis pain (Brief Pain Inventory [BPI]-Severity average pain score ≥4) receiving duloxetine 60 mg/day (n=177 analyzed) or placebo (n=176). Primary trial outcome was change from baseline in BPI-Severity average pain at Week 14. Subgroups included early pain reduction (≥30%, 10%-30%, or <10% decrease in BPI-Severity average pain at Week 4) and early TR-AESIs (with/without TR-AESIs by Week 2). Measures included changes from baseline in BPI-Severity average pain, QOL (BPI-Interference, Western Ontario and McMaster Universities Osteoarthritis Index), Patient Global Impression of Improvement (PGI-I), and response rate (proportion achieving ≥30% or ≥50% pain reduction at Week 14).
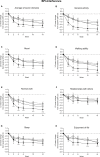
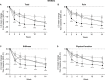
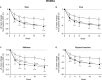
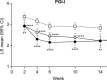
Results: The ≥30% early pain reduction subgroup (n=93) had significantly greater improvements in pain, QOL, and PGI-I and higher ≥30% and ≥50% response rates than placebo; the 10%-30% (n=45) and the <10% (n=33) pain reduction subgroups did not show the same (except 10%-30% group: PGI-I at Week 10 and some QOL at Weeks 10 and/or 14). Both TR-AESI subgroups (with, n=52; without, n=125) had significantly greater improvements in pain, PGI-I, and most QOL measures and higher response rates than placebo.
Conclusion: Early efficacy responses to duloxetine treatment, but not early TR-AESIs, may predict later pain reduction and QOL improvements in Japanese patients with knee osteoarthritis pain.
Clinicaltrialsgov: NCT02248480.
Keywords: Brief Pain Inventory; WOMAC; responder analysis.
Conflict of interest statement
Disclosure NI, TT, MI, and TO are full-time employees and minor stockholders in Shionogi & Co. Ltd. YU has received honoraria/consulting fees, travel support, fees for participation in review activities, fees for writing assistance, medicine, equipment, and administrative support, and/or grants from Shionogi & Co., Ltd; Astellas Pharma Inc.; Asahi Kasei Pharma Corporation; Chugai Pharmaceutical Co., Ltd; Daiichi Sankyo Co., Ltd; Kaken Pharmaceutical Co., Ltd; Nippon Zoki Pharmaceutical Co., Ltd; Seikagaku Corporation; Taisho Toyama Pharmaceutical Co., Ltd; Teijin Pharma Limited; Pfizer Japan Inc.; Japan Tissue Engineering Co., Ltd; Ayumi Pharmaceutical Corporation; Hisamitsu Pharmaceutical Co., Inc.; HOYA Technosurgical Corporation; Mochida Pharmaceutical Co., Ltd; Eli Lilly Japan K.K.; Olympus Terumo Biomaterials Corporation; Janssen Pharmaceutical K.K.; Ministry of Economy, Trade, and Industry; Japan Science and Technology Agency; Japan Society for the Promotion of Science; Japan Sports Medicine Foundation; and the Terumo Foundation for Life Sciences and Arts. SK has received grants, honoraria, reviewing fees, payment for lectures, fees for writing assistance, medicines, equipment, and/or administrative support from Shionogi & Co., Ltd; MSD K.K.; Asahi Kasei Pharma Corporation; Eli Lilly Japan K.K.; Eisai Co., Ltd; Ono Pharmaceutical Co., Ltd; Kaken Pharmaceutical Co., Ltd; Kowa Pharmaceutical Co., Ltd; Showa Yakuhin Kako Co., Ltd; Johnson & Johnson K.K.; Daiichi Sankyo Co., Ltd; Taisho Toyama Pharmaceutical Co., Ltd; Takeda Pharmaceutical Co., Ltd; Terumo Corporation; Nippon Zoki Pharmaceutical Co., Ltd; Hisamitsu Pharmaceutical Co., Inc.; Pfizer Japan Inc.; Janssen Pharmaceutical K.K.; Ayumi Pharmaceutical Corporation; Taiho Pharmaceutical Co., Ltd; Chugai Pharmaceutical Co., Ltd; Teijin Pharma Limited; Astellas Pharma Inc.; Nippon Shinyaku Co., Ltd; Stryker Japan K.K.; Tsumura & Co.; and Otsuka Pharmaceutical Co., Ltd. The authors report no other conflicts of interest in this work.
Figures


References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. - PMC - PubMed
-
- Muraki S, Oka H, Akune T, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage. 2009;17(9):1137–1143. - PubMed
-
- Xie F, Kovic B, Jin X, He X, Wang M, Silvestre C. Economic and humanistic burden of osteoarthritis: a systematic review of large sample studies. Pharmacoeconomics. 2016;34(11):1087–1100. - PubMed
-
- Vignon E, Valat JP, Rossignol M, et al. Osteoarthritis of the knee and hip and activity: a systematic international review and synthesis (OASIS) Joint Bone Spine. 2006;73(4):442–455. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

