Recent Advances in Visible-Light-Driven Photoelectrochemical Water Splitting: Catalyst Nanostructures and Reaction Systems
- PMID: 30464988
- PMCID: PMC6223929
- DOI: 10.1007/s40820-015-0063-3
Recent Advances in Visible-Light-Driven Photoelectrochemical Water Splitting: Catalyst Nanostructures and Reaction Systems
Abstract
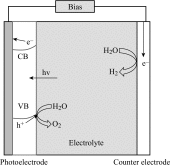
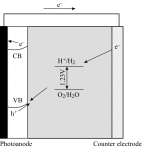
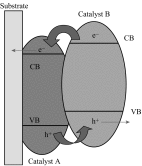
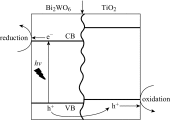
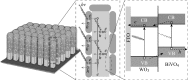
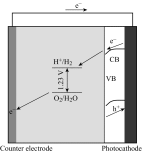
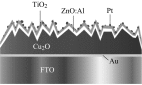
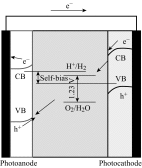
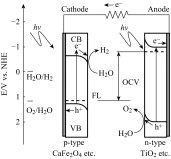
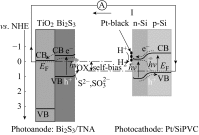
Photoelectrochemical (PEC) water splitting using solar energy has attracted great attention for generation of renewable hydrogen with less carbon footprint, while there are enormous challenges that still remain for improving solar energy water splitting efficiency, due to limited light harvesting, energy loss associated to fast recombination of photogenerated charge carriers, as well as electrode degradation. This overview focuses on the recent development about catalyst nanomaterials and nanostructures in different PEC water splitting systems. As photoanode, Au nanoparticle-decorated TiO2 nanowire electrodes exhibited enhanced photoactivity in both the UV and the visible regions due to surface plasmon resonance of Au and showed the largest photocurrent generation of up to 710 nm. Pt/CdS/CGSe electrodes were developed as photocathode. With the role of p-n heterojunction, the photoelectrode showed high stability and evolved hydrogen continuously for more than 10 days. Further, in the Z-scheme system (Bi2S3/TNA as photoanode and Pt/SiPVC as photocathode at the same time), a self-bias (open-circuit voltage V oc = 0.766 V) was formed between two photoelectrodes, which could facilitate photogenerated charge transfers and enhance the photoelectrochemical performance, and which might provide new hints for PEC water splitting. Meanwhile, the existing problems and prospective solutions have also been reviewed.
Keywords: Heterojuction; Hybrid systems; Nanostructures; Photoelectrochemical water splitting; Reaction system.
Figures





References
-
- Li Y, Zhang JZ. Hydrogen generation from photoelectrochemical water splitting based on nanomaterials. Laser Photonics Rev. 2010;4(4):517–528. doi: 10.1002/lpor.200910025. - DOI
-
- Kelly NA, Gibson TL. Design and characterization of a robust photoelectrochemical device to generate hydrogen using solar water splitting. Int. J. Hydrog. Energ. 2006;31(12):1658–1673. doi: 10.1016/j.ijhydene.2005.12.014. - DOI
-
- Minggu LJ, Daud WRW, Kassim MB. An overview of photocells and photoreactors for photoelectrochemical water splitting. Int. J. Hydrog. Energ. 2010;35(11):5233–5244. doi: 10.1016/j.ijhydene.2010.02.133. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
