NHE8 Deficiency Promotes Colitis-Associated Cancer in Mice via Expansion of Lgr5-Expressing Cells
- PMID: 30465020
- PMCID: PMC6240644
- DOI: 10.1016/j.jcmgh.2018.08.005
NHE8 Deficiency Promotes Colitis-Associated Cancer in Mice via Expansion of Lgr5-Expressing Cells
Abstract
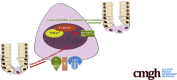
Background & aims: Lgr5 overexpression has been detected in colorectal cancers (CRCs), including some cases of colitis-associated CRCs. In colitis-associated CRCs, chronic inflammation is a contributing factor in carcinogenesis. We recently reported that intestinal Na+/H+ exchanger isoform 8 (NHE8) plays an important role in intestinal mucosal protection and that loss of NHE8 expression results in an ulcerative colitis-like condition. Therefore, we hypothesized that NHE8 may be involved in the development of intestinal tumors.
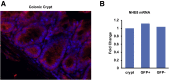
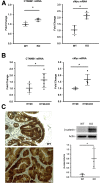
Methods: We assessed NHE8 expression in human CRCs by immunohistochemistry and studied tumor burden in NHE8 knockout (KO) mice using an azoxymethane/dextran sodium sulfate colon cancer model. We also evaluated cell proliferation in HT29NHE8KO cells and assessed tumor growth in NOD scid gamma (NSG) mice xenografted with HT29NHE8KO cells. To verify if a relationship exists between Lgr5 and NHE8 expression, we analyzed Lgr5 expression in NHE8KO mice by polymerase chain reaction and in situ hybridization. Lgr5 expression and cell proliferation in the absence of NHE8 were confirmed in colonic organoid cultures. The expression of β-catenin and c-Myc also were analyzed to evaluate Wnt/β-catenin activation.
Results: NHE8 was undetectable in human CRC tissues. Although only 9% of NHE8 wild-type mice showed tumorigenesis in the azoxymethane/dextran sodium sulfate colon cancer model, almost 10 times more NHE8KO mice (89%) developed tumors. In the absence of NHE8, a higher colony formation unit was discovered in HT29NHE8KO cells. In NSG mice, larger tumors developed at the site where HT29NHE8KO cells were injected compared with HT29NHE8 wild type cells. Furthermore, NHE8 deficiency resulted in increased Lgr5 expression in the colon, in HT29-derived tumors, and in colonoids. The absence of NHE8 also increased Wnt/β-catenin activation.
Conclusions: NHE8 might be an intrinsic factor that regulates Wnt/β-catenin in the intestine.
Keywords: AOM, azoxymethane; CRC, colorectal cancer; CRISPR/Cas9, clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9; Colorectal Tumor; DMEM, Dulbecco's modified Eagle medium; DSS, dextran sodium sulfate; EGFP, enhanced green fluorescent protein; EdU, 5-ethynyl-2’-deoxyuridine; FACS, fluorescence-activated cell sorter; KO, knockout; Lgr5; NHE, Na+/H+ exchanger; NHE8; NSG, NOD scid gamma; PCR, polymerase chain reaction; UC, ulcerative colitis; WT, wild type; mRNA, messenger RNA.
Figures





Comment in
-
Role of Membrane Transporters in Intestinal Cancers.Cell Mol Gastroenterol Hepatol. 2019;7(1):241-242. doi: 10.1016/j.jcmgh.2018.09.016. Epub 2018 Oct 28. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30585160 Free PMC article. No abstract available.
Similar articles
-
TRIB3 Interacts With β-Catenin and TCF4 to Increase Stem Cell Features of Colorectal Cancer Stem Cells and Tumorigenesis.Gastroenterology. 2019 Feb;156(3):708-721.e15. doi: 10.1053/j.gastro.2018.10.031. Epub 2018 Oct 24. Gastroenterology. 2019. PMID: 30365932
-
Tumor Necrosis Factor Ligand-Related Molecule 1A Regulates the Occurrence of Colitis-Associated Colorectal Cancer.Dig Dis Sci. 2018 Sep;63(9):2341-2350. doi: 10.1007/s10620-018-5126-0. Epub 2018 May 24. Dig Dis Sci. 2018. PMID: 29796912
-
Epithelial Smad4 Deletion Up-Regulates Inflammation and Promotes Inflammation-Associated Cancer.Cell Mol Gastroenterol Hepatol. 2018 May 24;6(3):257-276. doi: 10.1016/j.jcmgh.2018.05.006. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30109253 Free PMC article.
-
Molecular regulation mechanism of intestinal stem cells in mucosal injury and repair in ulcerative colitis.World J Gastroenterol. 2023 Apr 28;29(16):2380-2396. doi: 10.3748/wjg.v29.i16.2380. World J Gastroenterol. 2023. PMID: 37179583 Free PMC article. Review.
-
The intestinal stem cell as a target: A review.Medicine (Baltimore). 2024 Aug 23;103(34):e39456. doi: 10.1097/MD.0000000000039456. Medicine (Baltimore). 2024. PMID: 39183418 Free PMC article. Review.
Cited by
-
Application of CRISPR-Cas9 gene editing technology in basic research, diagnosis and treatment of colon cancer.Front Endocrinol (Lausanne). 2023 Mar 20;14:1148412. doi: 10.3389/fendo.2023.1148412. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37020597 Free PMC article. Review.
-
The Role of pHi in Intestinal Epithelial Proliferation-Transport Mechanisms, Regulatory Pathways, and Consequences.Front Cell Dev Biol. 2021 Jan 22;9:618135. doi: 10.3389/fcell.2021.618135. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33553180 Free PMC article. Review.
-
Emerging links between endosomal pH and cancer.Cancer Metastasis Rev. 2020 Jun;39(2):519-534. doi: 10.1007/s10555-020-09870-1. Cancer Metastasis Rev. 2020. PMID: 32253638 Free PMC article. Review.
-
How Dysregulated Ion Channels and Transporters Take a Hand in Esophageal, Liver, and Colorectal Cancer.Rev Physiol Biochem Pharmacol. 2021;181:129-222. doi: 10.1007/112_2020_41. Rev Physiol Biochem Pharmacol. 2021. PMID: 32875386 Review.
-
Downregulation of SLC9A8 Promotes Epithelial-Mesenchymal Transition and Metastasis in Colorectal Cancer Cells via the IL6-JAK1/STAT3 Signaling Pathway.Dig Dis Sci. 2023 May;68(5):1873-1884. doi: 10.1007/s10620-022-07805-0. Epub 2022 Dec 30. Dig Dis Sci. 2023. PMID: 36583805
References
-
- Orlowski J., Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. - PubMed
-
- Zachos N.C., Tse M., Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005;67:411–443. - PubMed
-
- Xu H., Chen H., Dong J., Lynch R., Ghishan F.K. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8) Cell Physiol Biochem. 2008;21:109–116. - PubMed
-
- Xu H., Chen R., Ghishan F.K. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol. 2005;289:G36–G41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

