TIA1 regulates the generation and response to toxic tau oligomers
- PMID: 30465259
- PMCID: PMC6377165
- DOI: 10.1007/s00401-018-1937-5
TIA1 regulates the generation and response to toxic tau oligomers
Abstract
RNA binding proteins (RBPs) are strongly linked to the pathophysiology of motor neuron diseases. Recent studies show that RBPs, such as TIA1, also contribute to the pathophysiology of tauopathy. RBPs co-localize with tau pathology, and reduction of TIA1 protects against tau-mediated neurodegeneration. The mechanism through which TIA1 reduction protects against tauopathy, and whether TIA1 modulates the propagation of tau, are unknown. Previous studies indicate that the protective effect of TIA1 depletion correlates with both the reduction of oligomeric tau and the reduction of pathological TIA1 positive tau inclusions. In the current report, we used a novel tau propagation approach to test whether TIA1 is required for producing toxic tau oligomers and whether TIA1 reduction would provide protection against the spread of these oligomers. The approach used young PS19 P301S tau mice at an age at which neurodegeneration would normally not yet occur and seeding oligomeric or fibrillar tau by injection into hippocampal CA1 region. We find that propagation of exogenous tau oligomers (but not fibrils) across the brain drives neurodegeneration in this model. We demonstrate that TIA1 reduction essentially brackets the pathophysiology of tau, being required for the production of tau oligomers, as well as regulating the response of neurons to propagated toxic tau oligomers. These results indicate that RNA binding proteins modulate the pathophysiology of tau at multiple levels and provide insights into possible therapeutic approaches to reduce the spread of neurodegeneration in tauopathy.
Keywords: Neurodegeneration; Neuropathology; RNA binding proteins; Stress granules; TIA1; Tau fibrils; Tau oligomers; Tau propagation; Tauopathy.
Conflict of interest statement
BW is co-founder and Chief Scientific Officer for Aquinnah Pharmaceuticals Inc.
Figures


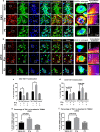

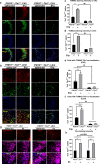


References
-
- Ahmed Z, Cooper J, Murray TK, Garn K, Mcnaughton E, Clarke H, et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;3:667–683. doi: 10.1007/s00401-014-1254-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

