Metazoan evolution of glutamate receptors reveals unreported phylogenetic groups and divergent lineage-specific events
- PMID: 30465522
- PMCID: PMC6307864
- DOI: 10.7554/eLife.35774
Metazoan evolution of glutamate receptors reveals unreported phylogenetic groups and divergent lineage-specific events
Abstract
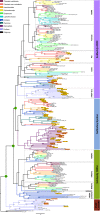
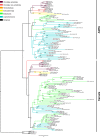
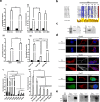
Glutamate receptors are divided in two unrelated families: ionotropic (iGluR), driving synaptic transmission, and metabotropic (mGluR), which modulate synaptic strength. The present classification of GluRs is based on vertebrate proteins and has remained unchanged for over two decades. Here we report an exhaustive phylogenetic study of GluRs in metazoans. Importantly, we demonstrate that GluRs have followed different evolutionary histories in separated animal lineages. Our analysis reveals that the present organization of iGluRs into six classes does not capture the full complexity of their evolution. Instead, we propose an organization into four subfamilies and ten classes, four of which have never been previously described. Furthermore, we report a sister class to mGluR classes I-III, class IV. We show that many unreported proteins are expressed in the nervous system, and that new Epsilon receptors form functional ligand-gated ion channels. We propose an updated classification of glutamate receptors that includes our findings.
Keywords: amphioxus; electrophysiology; evolutionary biology; gene expression; ionotropic glutamate receptors; metabotropic glutamate receptors; neuroscience; phylogenetics.
© 2018, Ramos-Vicente et al.
Conflict of interest statement
DR, JJ, EG, GG, RR, JL, DB, EN, JG, PF, HE, NR, DS, ÀB No competing interests declared
Figures






















Comment in
-
Family matters.Elife. 2018 Dec 28;7:e43815. doi: 10.7554/eLife.43815. Elife. 2018. PMID: 30592460 Free PMC article.
References
-
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Research. 2014;42:W252–W258. doi: 10.1093/nar/gku340. - DOI - PMC - PubMed

