PAQR3 Regulates Endoplasmic Reticulum-to-Golgi Trafficking of COPII Vesicle via Interaction with Sec13/Sec31 Coat Proteins
- PMID: 30466064
- PMCID: PMC6249397
- DOI: 10.1016/j.isci.2018.11.002
PAQR3 Regulates Endoplasmic Reticulum-to-Golgi Trafficking of COPII Vesicle via Interaction with Sec13/Sec31 Coat Proteins
Abstract
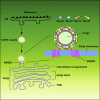
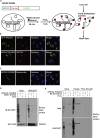
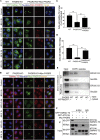
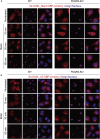
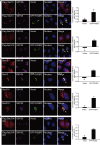
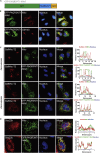
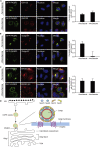
Endoplasmic reticulum (ER)-to-Golgi anterograde transport is driven by COPII vesicles mainly composed of a Sec23/Sec24 inner shell and a Sec13/Sec31 outer cage. How COPII vesicles are tethered to the Golgi is not completely understood. We demonstrated here that PAQR3 can facilitate tethering of COPII vesicles to the Golgi. Proximity labeling using PAQR3 fused with APEX2 identified that many proteins involved in intracellular transport are in close proximity to PAQR3. ER-to-Golgi trafficking of N-acetylgalactosaminyltransferase-2 on removal of brefeldin A is delayed by PAQR3 deletion. RUSH assay also revealed that ER-to-Golgi trafficking is affected by PAQR3. The N-terminal end of PAQR3 can interact with the WD domains of Sec13 and Sec31A. PAQR3 enhances Golgi localization of Sec13 and Sec31A. Furthermore, PAQR3 is localized in the ERGIC and cis-Golgi structures, the acceptor sites for COPII vesicles. Taken together, our study uncovers a role for PAQR3 as a player in regulating ER-to-Golgi transport of COPII vesicles.
Keywords: Cell Biology; Functional Aspects of Cell Biology; Molecular Biology Experimental Approach.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
p125A exists as part of the mammalian Sec13/Sec31 COPII subcomplex to facilitate ER-Golgi transport.J Cell Biol. 2010 Aug 9;190(3):331-45. doi: 10.1083/jcb.201003005. Epub 2010 Aug 2. J Cell Biol. 2010. PMID: 20679433 Free PMC article.
-
The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the golgi.Cold Spring Harb Perspect Biol. 2013 Feb 1;5(2):a013367. doi: 10.1101/cshperspect.a013367. Cold Spring Harb Perspect Biol. 2013. PMID: 23378591 Free PMC article. Review.
-
ALG-2 attenuates COPII budding in vitro and stabilizes the Sec23/Sec31A complex.PLoS One. 2013 Sep 19;8(9):e75309. doi: 10.1371/journal.pone.0075309. eCollection 2013. PLoS One. 2013. PMID: 24069399 Free PMC article.
-
Carbon tetrachloride suppresses ER-Golgi transport by inhibiting COPII vesicle formation on the ER membrane in the RLC-16 hepatocyte cell line.Cell Biol Int. 2021 Mar;45(3):633-641. doi: 10.1002/cbin.11510. Epub 2020 Dec 8. Cell Biol Int. 2021. PMID: 33247607
-
New insights into the structural mechanisms of the COPII coat.Traffic. 2010 Mar;11(3):303-10. doi: 10.1111/j.1600-0854.2009.01026.x. Epub 2009 Dec 7. Traffic. 2010. PMID: 20070605 Review.
Cited by
-
In Vivo Proximity Labeling Identifies a New Function for the Lifespan and Autophagy-regulating Kinase Pef1, an Ortholog of Human Cdk5.bioRxiv [Preprint]. 2024 Jun 14:2024.06.12.598664. doi: 10.1101/2024.06.12.598664. bioRxiv. 2024. PMID: 38915521 Free PMC article. Preprint.
-
NBEAL1 controls SREBP2 processing and cholesterol metabolism and is a susceptibility locus for coronary artery disease.Sci Rep. 2020 Mar 11;10(1):4528. doi: 10.1038/s41598-020-61352-0. Sci Rep. 2020. PMID: 32161285 Free PMC article.
-
Deciphering Spatial Protein-Protein Interactions in Brain Using Proximity Labeling.Mol Cell Proteomics. 2022 Nov;21(11):100422. doi: 10.1016/j.mcpro.2022.100422. Epub 2022 Oct 2. Mol Cell Proteomics. 2022. PMID: 36198386 Free PMC article. Review.
-
Proximity labeling in mammalian cells with TurboID and split-TurboID.Nat Protoc. 2020 Dec;15(12):3971-3999. doi: 10.1038/s41596-020-0399-0. Epub 2020 Nov 2. Nat Protoc. 2020. PMID: 33139955
-
Nlp-dependent ER-to-Golgi transport.Int J Biol Sci. 2024 May 11;20(8):2881-2903. doi: 10.7150/ijbs.91792. eCollection 2024. Int J Biol Sci. 2024. PMID: 38904019 Free PMC article.
References
LinkOut - more resources
Full Text Sources

