A Pharmacogenomic-based Antidepressant Treatment for Patients with Major Depressive Disorder: Results from an 8-week, Randomized, Single-blinded Clinical Trial
- PMID: 30466219
- PMCID: PMC6245286
- DOI: 10.9758/cpn.2018.16.4.469
A Pharmacogenomic-based Antidepressant Treatment for Patients with Major Depressive Disorder: Results from an 8-week, Randomized, Single-blinded Clinical Trial
Erratum in
-
Corrigendum: A Pharmacogenomic-based Antidepressant Treatment for Patients with Major Depressive Disorder: Results from an 8-week, Randomized, Single-blinded Clinical Trial.Clin Psychopharmacol Neurosci. 2020 Nov 30;18(4):641. doi: 10.9758/cpn.2020.18.4.641. Clin Psychopharmacol Neurosci. 2020. PMID: 33124599 Free PMC article. No abstract available.
Abstract
Objective: Pharmacogenomic-based antidepressant treatment (PGATx) may result in more precise pharmacotherapy of major depressive disorder (MDD) with better drug therapy guidance.
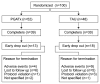
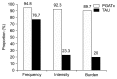
Methods: An 8-week, randomized, single-blind clinical trial was conducted to evaluate the effectiveness and tolerability of PGATx in 100 patients with MDD. All recruited patients were randomly allocated either to PGATx (n=52) or treatment as usual (TAU, n=48) groups. The primary endpoint was a change of total score of the Hamilton Depression Rating Scale-17 (HAMD-17) from baseline to end of treatment. Response rate (at least 50% reduction in HAMD-17 score from baseline), remission rate (HAMD-17 score ≤7 at the end of treatment) as well as the change of total score of Frequency, Intensity, and Burden of Side Effects Ratings (FIBSER) from baseline to end of treatment were also investigated.
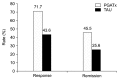
Results: The mean change of HAMD-17 score was significantly different between two groups favoring PGATx by -4.1 point of difference (p=0.010) at the end of treatment. The mean change in the FIBSER score from baseline was significantly different between two treatment groups favoring PGATx by -2.5 point of difference (p=0.028). The response rate (71.7 % vs. 43.6%, p=0.014) were also significantly higher in PGATx than in TAU at the end of treatment, while the remission rate was numerically higher in PGATx than in TAU groups without statistical difference (45.5% vs. 25.6%, p=0.071). The reason for early drop-out associated with adverse events was also numerically higher in TAU (n=9, 50.0%) than in PGATx (n=4, 30.8%).
Conclusion: The present study clearly demonstrate that PGATx may be a better treatment option in the treatment of MDD in terms of effectiveness and tolerability; however, study shortcomings may limit a generalization. Adequately-powered, well-designed, subsequent studies should be mandatory to prove its practicability and clinical utility for routine practice.
Keywords: Antidepressants; Depressive disorder; Effects; Pharmacogenetic testing; Precision medicine; Tolerance.
Figures
References
LinkOut - more resources
Full Text Sources
Medical

