BRD4 and Cancer: going beyond transcriptional regulation
- PMID: 30466442
- PMCID: PMC6251205
- DOI: 10.1186/s12943-018-0915-9
BRD4 and Cancer: going beyond transcriptional regulation
Abstract
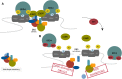
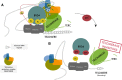
BRD4, member of the Bromodomain and Extraterminal (BET) protein family, is largely acknowledged in cancer for its role in super-enhancers (SEs) organization and oncogenes expression regulation. Inhibition of BRD4 shortcuts the communication between SEs and target promoters with a subsequent cell-specific repression of oncogenes to which cancer cells are addicted and cell death. To date, this is the most credited mechanism of action of BET inhibitors, a class of small molecules targeting BET proteins which are currently in clinical trials in several cancer settings.However, recent evidence indicates that BRD4 relevance in cancer goes beyond its role in transcription regulation and identifies this protein as a keeper of genome stability.Indeed, a non-transcriptional role of BRD4 in controlling DNA damage checkpoint activation and repair as well as telomere maintenance has been proposed, throwing new lights into the multiple functions of this protein and opening new perspectives on the use of BETi in cancer. Here we discuss the current available information on non-canonical, non-transcriptional functions of BRD4 and on their implications in cancer biology. Integrating this information with the already known BRD4 role in gene expression regulation, we propose a "common" model to explain BRD4 genomic function. Furthermore, in light of the transversal function of BRD4, we provide new interpretation for the cytotoxic activity of BETi and we discuss new possibilities for a wide and focused employment of these drugs in clinical settings.
Keywords: BET inhibitors; BRD4; Cancer; DNA damage response; Telomere regulation; Transcriptional regulation; Unconventional function.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

