Targeting the Pentose Phosphate Pathway: Characterization of a New 6PGL Inhibitor
- PMID: 30467026
- PMCID: PMC6289167
- DOI: 10.1016/j.bpj.2018.10.027
Targeting the Pentose Phosphate Pathway: Characterization of a New 6PGL Inhibitor
Abstract
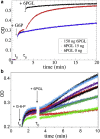
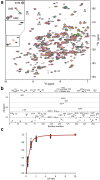
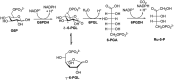
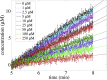
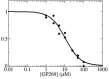
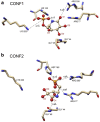
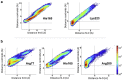
Human African trypanosomiasis, or sleeping sickness, is a lethal disease caused by the protozoan parasite Trypanosoma brucei. However, although many efforts have been made to understand the biochemistry of this parasite, drug development has led to treatments that are of limited efficiency and of great toxicity. To develop new drugs, new targets must be identified, and among the several metabolic processes of trypanosomes that have been proposed as drug targets, carbohydrate metabolism (glycolysis and the pentose phosphate pathway (PPP)) appears as a promising one. As far as the PPP is concerned, a limited number of studies are related to the glucose-6-phosphate dehydrogenase. In this work, we have focused on the activity of the second PPP enzyme (6-phospho-gluconolactonase (6PGL)) that transforms 6-phosphogluconolactone into 6-phosphogluconic acid. A lactam analog of the natural substrate has been synthesized, and binding of the ligand to 6PGL has been investigated by NMR titration. The ability of this ligand to inhibit 6PGL has also been demonstrated using ultraviolet experiments, and protein-inhibitor interactions have been investigated through docking calculations and molecular dynamics simulations. In addition, a marginal inhibition of the third enzyme of the PPP (6-phosphogluconate dehydrogenase) was also demonstrated. Our results thus open new prospects for targeting T. brucei.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
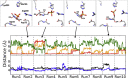
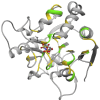
References
-
- Barrett M.P., Burchmore R.J., Krishna S. The trypanosomiases. Lancet. 2003;362:1469–1480. - PubMed
-
- Funayama S., Funayama S., Veiga L.A. Trypanosoma cruzi: kinetic properties of glucose-6-phosphate dehydrogenase. Exp. Parasitol. 1977;43:376–381. - PubMed
-
- Büscher P., Cecchi G., Priotto G. Human African trypanosomiasis. Lancet. 2017;390:2397–2409. - PubMed
-
- Opperdoes F.R. Compartmentation of carbohydrate metabolism in trypanosomes. Annu. Rev. Microbiol. 1987;41:127–151. - PubMed
-
- Kovářová J., Barrett M.P. The pentose phosphate pathway in parasitic trypanosomatids. Trends Parasitol. 2016;32:622–634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

