Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair
- PMID: 30467144
- PMCID: PMC6684198
- DOI: 10.1126/science.aar2971
Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair
Abstract
During tissue repair, myofibroblasts produce extracellular matrix (ECM) molecules for tissue resilience and strength. Altered ECM deposition can lead to tissue dysfunction and disease. Identification of distinct myofibroblast subsets is necessary to develop treatments for these disorders. We analyzed profibrotic cells during mouse skin wound healing, fibrosis, and aging and identified distinct subpopulations of myofibroblasts, including adipocyte precursors (APs). Multiple mouse models and transplantation assays demonstrate that proliferation of APs but not other myofibroblasts is activated by CD301b-expressing macrophages through insulin-like growth factor 1 and platelet-derived growth factor C. With age, wound bed APs and differential gene expression between myofibroblast subsets are reduced. Our findings identify multiple fibrotic cell populations and suggest that the environment dictates functional myofibroblast heterogeneity, which is driven by fibroblast-immune interactions after wounding.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


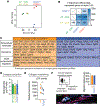

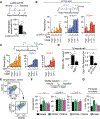
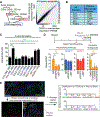
Comment in
-
Cellular networks in wound healing.Science. 2018 Nov 23;362(6417):891-892. doi: 10.1126/science.aav5542. Science. 2018. PMID: 30467155 No abstract available.
References
-
- Gosain A, DiPietro LA, Aging and wound healing. World J Surg. 28, 321–326 (2004). - PubMed
-
- Ballas CB, Davidson JM, Delayed wound healing in aged rats is associated with increased collagen gel remodeling and contraction by skin fibroblasts, not with differences in apoptotic or myofibroblast cell populations. Wound Repair Regen. 9, 223–237 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

