First-line therapy in chronic lymphocytic leukemia: a Swedish nation-wide real-world study on 1053 consecutive patients treated between 2007 and 2013
- PMID: 30467205
- PMCID: PMC6442960
- DOI: 10.3324/haematol.2018.200204
First-line therapy in chronic lymphocytic leukemia: a Swedish nation-wide real-world study on 1053 consecutive patients treated between 2007 and 2013
Abstract
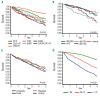
The aim of this study was to investigate long-term outcome following first-line therapy in consecutive chronic lymphocytic leukemia (CLL) patients in a well-defined geographic area: Sweden. All patients diagnosed with CLL (2007-2013) (n=3672) were identified from national registries, screening of patient files identified all (100%) treated first line (n=1053) and for those, an in-depth analysis was performed. End points were overall response rate, progression-free survival (PFS), overall survival (OS), and safety. Median age was 71 years; 53% had Rai stage III-IV and 97% had performance status grade 0-2. Fluorescence in situ hybridization (FISH) was performed in 57% of patients: 15% had del(17p). Chlorambucil + prednisone was used in 39% (5% also received rituximab). Fludarabine+cyclophosphamide+rituximab or fludarabine+cyclophosphamide was used in 43% and bendamustine + rituximab in 6%. Overall response rate was 64%; chlorambucil 43%, fludarabine+cyclophosphamide+rituximab 84%, fludarabine+cyclophosphamide 75% and bendamustine + rituximab 75%. Median PFS and OS was 24 and 58 months, respectively, both were significantly associated (multivariate analysis) with type of treatment, del(17p), performance status, gender, age and geographical region (OS only). Chlorambucil-treated patients had a median PFS and OS of only 9 and 33 months, respectively. Chlorambucil usage declined gradually throughout the study period, but one-third of patients still received chlorambucil + rituximab in 2013. Infections ≥grade III were significantly associated with treatment; chlorambucil 19% versus fludarabine+cyclophosphamide+rituximab 30%. Richter transformation occurred in 5.5% of the patients, equally distributed across therapies. This is the largest retrospective, real-world cohort of consecutive first-line treated CLL patients with a complete follow up. In elderly patients, an unmet need for more effective, well-tolerated therapies was identified.
Copyright© 2019 Ferrata Storti Foundation.
Figures

References
-
- Asklid A, Winqvist M, Eketorp Sylvan S, et al. Outcomes of second-line treatment in chronic lymphocytic leukemia - a population-based study from a well defined geographical region between 2003 and 2013. Leuk Lymphoma. 2017;58(5):1219–1223. - PubMed
-
- Eichhorst B, Dreyling M, Robak T, et al. Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi50–54. - PubMed
-
- Svenska KLL gruppen https://www.cancer-centrum.se/syd/cancerdiagnoser/blod-lym-fom-myelom/kr... Regionalt cancercentrum Stockholm och Gotland. 2016.
-
- Siegel RL, Sahar L, Portier KM, Ward EM, Jemal A. Cancer death rates in US congressional districts. CA Cancer J Clin. 2015;65(5):339–344. - PubMed
-
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for diagnosis, indications for treatment, response assessment and supportive management of chronic lymphocytic leukemia. Blood. 2018;131(25):2745–2760. - PubMed

