Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes
- PMID: 30467295
- PMCID: PMC6320976
- DOI: 10.3390/ijms19123720
Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes
Abstract
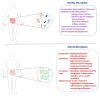
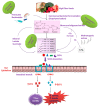
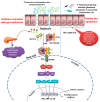
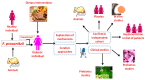
The incidence of metabolic disorders, including diabetes, has elevated exponentially during the last decades and enhanced the risk of a variety of complications, such as diabetes and cardiovascular diseases. In the present review, we have highlighted the new insights on the complex relationships between diet-induced modulation of gut microbiota and metabolic disorders, including diabetes. Literature from various library databases and electronic searches (ScienceDirect, PubMed, and Google Scholar) were randomly collected. There exists a complex relationship between diet and gut microbiota, which alters the energy balance, health impacts, and autoimmunity, further causes inflammation and metabolic dysfunction, including diabetes. Faecalibacterium prausnitzii is a butyrate-producing bacterium, which plays a vital role in diabetes. Transplantation of F. prausnitzii has been used as an intervention strategy to treat dysbiosis of the gut's microbial community that is linked to the inflammation, which precedes autoimmune disease and diabetes. The review focuses on literature that highlights the benefits of the microbiota especially, the abundant of F. prausnitzii in protecting the gut microbiota pattern and its therapeutic potential against inflammation and diabetes.
Keywords: Faecalibacterium prausnitzii; diabetes; diet; gut microbiota; novel strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

