Lipid Metabolism Alteration by Endocrine Disruptors in Animal Models: An Overview
- PMID: 30467492
- PMCID: PMC6236061
- DOI: 10.3389/fendo.2018.00654
Lipid Metabolism Alteration by Endocrine Disruptors in Animal Models: An Overview
Abstract
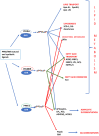
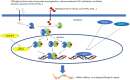
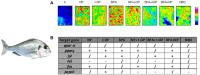
Exposure to potential Endocrine Disrupting Chemicals (EDCs) pose a documented risk to both wildlife and human health. Many studies so far described declining sperm counts, genital malformations, early puberty onset, highlighting the negative impact on reproduction caused by the exposure to many anthropogenic chemicals. In the last years, increasing evidence suggested that these compounds, other than altering reproduction, affect metabolism and induce the onset of obesity and metabolic disorders. According to the "environmental obesogens" hypothesis, evidence exists that exposure to potential EDCs during critical periods when adipocytes are differentiating, and organs are developing, can induce diseases that manifest later in the life. This review summarizes the effects occurring at the hepatic level in different animal models, describing morphological alterations and changes of molecular pathways elicited by the toxicant exposure. Results currently available demonstrated that these chemicals impair normal metabolic processes via interaction with members of the nuclear receptor superfamily, including steroid hormone receptors, thyroid hormone receptors, retinoid X receptors, peroxisome proliferator-activated receptors, liver X receptors, and farnesoid X receptors. In addition, novel results revealed that EDC exposure can either affect circadian rhythms as well as up-regulate the expression of signals belonging to the endocannabinoid system, in both cases leading to a remarkable increase of lipid accumulation. These results warrant further research and increase the interest toward the identification of new mechanisms for EDC metabolic alterations. The last part of this review article condenses recent evidences on the ability of potential EDCs to cause "transgenerational effects" by a single prenatal or early life exposure. On this regard, there is compelling evidence that epigenetic modifications link developmental environmental insults to adult disease susceptibility. This review will contribute to summarize the mechanisms underlying the insurgence of EDC-induced metabolic alterations as well as to build integrated strategies for their better management. In fact, despite the large number of results obtained so far, there is still a great demand for the development of frameworks that can integrate mechanistic and toxicological/epidemiological observations. This would increase legal and governmental institution awareness on this critical environmental issue responsible for negative consequences in both wild species and human health.
Keywords: epigenetic; metabolic disorders; phthalates; reproduction; zebrafish (Danio rerio).
Figures
References
-
- Street ME, Angelini S, Bernasconi S, Burgio E, Cassio A, Catellani C, et al. . Current knowledge on endocrine disrupting chemicals (EDCs) from animal biology to humans , from pregnancy to adulthood : highlights from a national italian meeting. Int J Mol Sci. (2018) 19:1647. 10.3390/ijms19061647 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

