Effects of Monolaurin on Oral Microbe-Host Transcriptome and Metabolome
- PMID: 30467497
- PMCID: PMC6237204
- DOI: 10.3389/fmicb.2018.02638
Effects of Monolaurin on Oral Microbe-Host Transcriptome and Metabolome
Abstract
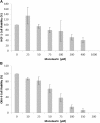
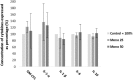
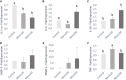
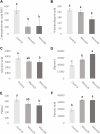
The aim of this in vitro study was to evaluate the effects of monolaurin against Aggregatibacter actinomycetemcomitans (Aa) and determine their effects on the host transcriptome and metabolome, using an oral cell/bacteria co-culture dual-chamber model to mimic the human periodontium. For this, the Aa, was applied to cross the monolayer of epithelial keratinocytes (OBA-9) to reach the fibroblasts layer (HGF-1) in the basal chamber. The Monolaurin treatments (25 or 50 μM) were added immediately after the inoculation of the dual-chamber with Aa. After 24 h, the transcriptional factors and metabolites produced were quantified in the remaining cell layers (insert and basal chamber) and in supernatant released from the cells. The genes IL-1α, IL-6, IL-18, and TNF analyzed in HGF-1 concentrations showed a decreased expression when treated with both concentration of Monolaurin. In keratinocytes, the genes IL-6, IL-18, and TNF presented a higher expression and the expression of IL-1α decreased when treated with the two cited concentrations. The production of glycerol and pyruvic acid increased, and the 2-deoxytetronic acid NIST, 4-aminobutyric acid, pinitol and glyceric acid, presented lower concentrations because of the treatment with 25 and/or 50 μM of Monolaurin. Use of monolaurin modulated the immune response and metabolite production when administered for 24 h in a dual-chamber model inoculated with A. actinomycetemcomitans. In summary, this study indicates that monolaurin had antimicrobial activity and modulated the host immune response and metabolite production when administered for 24 h in a dual-chamber model inoculated with A. actinomycetemcomitans.
Keywords: Aggregatibacter actinomycetemcomitans; fibroblast; immune system; keratinocyte; monolaurin; periodontal disease.
Figures

Similar articles
-
Oral microbe-host interactions: influence of β-glucans on gene expression of inflammatory cytokines and metabolome profile.BMC Microbiol. 2017 Mar 7;17(1):53. doi: 10.1186/s12866-017-0946-1. BMC Microbiol. 2017. PMID: 28270109 Free PMC article.
-
Malva sylvestris Inhibits Inflammatory Response in Oral Human Cells. An In Vitro Infection Model.PLoS One. 2015 Oct 19;10(10):e0140331. doi: 10.1371/journal.pone.0140331. eCollection 2015. PLoS One. 2015. PMID: 26479870 Free PMC article.
-
In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms.PeerJ. 2016 Jun 22;4:e2148. doi: 10.7717/peerj.2148. eCollection 2016. PeerJ. 2016. PMID: 27366648 Free PMC article.
-
Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis.Mol Oral Microbiol. 2016 Jun;31(3):207-27. doi: 10.1111/omi.12119. Epub 2015 Sep 22. Mol Oral Microbiol. 2016. PMID: 26197893 Free PMC article. Review.
-
Virulence factors of Actinobacillus actinomycetemcomitans.Periodontol 2000. 1999 Jun;20:136-67. doi: 10.1111/j.1600-0757.1999.tb00161.x. Periodontol 2000. 1999. PMID: 10522226 Review.
Cited by
-
Yeast-Host Interactions: Anadenanthera colubrina Modulates Virulence Factors of C. albicans and Inflammatory Response In Vitro.Front Pharmacol. 2021 Jun 8;12:629778. doi: 10.3389/fphar.2021.629778. eCollection 2021. Front Pharmacol. 2021. PMID: 34168555 Free PMC article.
-
Monolaurin inhibits antibiotic-resistant Staphylococcus aureus in patients with atopic dermatitis.Sci Rep. 2025 Jul 2;15(1):23180. doi: 10.1038/s41598-025-05667-w. Sci Rep. 2025. PMID: 40604030 Free PMC article.
-
Effect of mouthwash containing poly l-Lysine and glycerol monolaurate on oral Helicobacter pylori relating to biofilm eradication, anti-adhesion, and pro-inflammatory cytokine suppression.J Dent Sci. 2024 Jul;19(3):1748-1757. doi: 10.1016/j.jds.2023.10.010. Epub 2023 Oct 21. J Dent Sci. 2024. PMID: 39035281 Free PMC article.
-
Glycerol Monolaurate Ameliorated Intestinal Barrier and Immunity in Broilers by Regulating Intestinal Inflammation, Antioxidant Balance, and Intestinal Microbiota.Front Immunol. 2021 Sep 23;12:713485. doi: 10.3389/fimmu.2021.713485. eCollection 2021. Front Immunol. 2021. PMID: 34630388 Free PMC article.
-
Postbiotics enhance the efficacy of derivative compound mouthwash against clinical Helicobacter pylori strains.Front Cell Infect Microbiol. 2025 Jul 23;15:1629106. doi: 10.3389/fcimb.2025.1629106. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40771311 Free PMC article.
References
-
- Aimetti M. (2014). Nonsurgical periodontal treatment. Int. J. Esthet. Dent. 9 251–267. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

