Making CAR T Cells a Solid Option for Solid Tumors
- PMID: 30467505
- PMCID: PMC6235951
- DOI: 10.3389/fimmu.2018.02593
Making CAR T Cells a Solid Option for Solid Tumors
Abstract
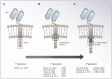
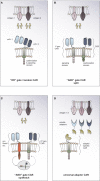
Adoptive cell therapy with chimeric antigen receptor (CAR) T cells aims to redirect the patient's own immune system to selectively attack cancer cells. To do so, CAR T cells are endowed with specific antigen recognition moieties fused to signaling and costimulatory domains. While this approach has shown great success for the treatment of B cell malignancies, response rates among patients with solid cancers are less favorable. The major challenges for CAR T cell immunotherapy in solid cancers are the identification of unique tumor target antigens, as well as improving CAR T cell trafficking to and expansion at the tumor site. This review focuses on combinatorial antigen targeting, regional delivery and approaches to improve CAR T cell persistence in the face of a hostile tumor microenvironment.
Keywords: CAR-T cells; cancer; cell engineering; immunotherapy; solid tumors; toxicity.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

