Genetic Mutations in jamb, jamc, and myomaker Revealed Different Roles on Myoblast Fusion and Muscle Growth
- PMID: 30467785
- PMCID: PMC6467518
- DOI: 10.1007/s10126-018-9865-x
Genetic Mutations in jamb, jamc, and myomaker Revealed Different Roles on Myoblast Fusion and Muscle Growth
Abstract
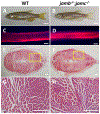
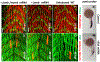
Myoblast fusion is a vital step for skeletal muscle development, growth, and regeneration. Loss of Jamb, Jamc, or Myomaker (Mymk) function impaired myoblast fusion in zebrafish embryos. In addition, mymk mutation hampered fish muscle growth. However, the effect of Jamb and Jamc deficiency on fish muscle growth is not clear. Moreover, whether jamb;jamc and jamb;mymk double mutations have stronger effects on myoblast fusion and muscle growth remains to be investigated. Here, we characterized the muscle development and growth in jamb, jamc, and mymk single and double mutants in zebrafish. We found that although myoblast fusion was compromised in jamb and jamc single or jamb;jamc double mutants, these mutant fish showed no defect in muscle cell fusion during muscle growth. The mutant fish were able to grow into adults that were indistinguishable from the wild-type sibling. In contrast, the jamb;mymk double mutants exhibited a stronger muscle phenotype compared to the jamb and jamc single and double mutants. The jamb;mymk double mutant showed reduced growth and partial lethality, similar to a mymk single mutant. Single fiber analysis of adult skeletal myofibers revealed that jamb, jamc, or jamb;jamc mutants contained mainly multinucleated myofibers, whereas jamb;mymk double mutants contained mostly mononucleated fibers. Significant intramuscular adipocyte infiltration was found in skeletal muscles of the jamb;mymk mutant. Collectively, these studies demonstrate that although Jamb, Jamc, and Mymk are all involved in myoblast fusion during early myogenesis, they have distinct roles in myoblast fusion during muscle growth. While Mymk is essential for myoblast fusion during both muscle development and growth, Jamb and Jamc are dispensable for myoblast fusion during muscle growth.
Keywords: Jamb; Jamc; Muscle fusion; Myomaker; Zebrafish.
Conflict of interest statement
Conflict of Interest Statement
All authors declared no conflict of interest.
Figures






Similar articles
-
The regulatory role of Myomaker and Myomixer-Myomerger-Minion in muscle development and regeneration.Cell Mol Life Sci. 2020 Apr;77(8):1551-1569. doi: 10.1007/s00018-019-03341-9. Epub 2019 Oct 23. Cell Mol Life Sci. 2020. PMID: 31642939 Free PMC article. Review.
-
Knockout of myomaker results in defective myoblast fusion, reduced muscle growth and increased adipocyte infiltration in zebrafish skeletal muscle.Hum Mol Genet. 2018 Oct 15;27(20):3542-3554. doi: 10.1093/hmg/ddy268. Hum Mol Genet. 2018. PMID: 30016436
-
Cell fusion is differentially regulated in zebrafish post-embryonic slow and fast muscle.Dev Biol. 2020 Jun 1;462(1):85-100. doi: 10.1016/j.ydbio.2020.03.005. Epub 2020 Mar 10. Dev Biol. 2020. PMID: 32165147 Free PMC article.
-
A defect in myoblast fusion underlies Carey-Fineman-Ziter syndrome.Nat Commun. 2017 Jul 6;8:16077. doi: 10.1038/ncomms16077. Nat Commun. 2017. PMID: 28681861 Free PMC article.
-
[Molecular regulation mechanism of Myomaker and Myomerger in myoblast fusion].Yi Chuan. 2019 Dec 20;41(12):1110-1118. doi: 10.16288/j.yczz.19-232. Yi Chuan. 2019. PMID: 31857282 Review. Chinese.
Cited by
-
Temporal Expression of Myogenic Regulatory Genes in Different Chicken Breeds during Embryonic Development.Int J Mol Sci. 2022 Sep 4;23(17):10115. doi: 10.3390/ijms231710115. Int J Mol Sci. 2022. PMID: 36077516 Free PMC article.
-
Direct Phenotyping and Principal Component Analysis of Type Traits Implicate Novel QTL in Bovine Mastitis through Genome-Wide Association.Animals (Basel). 2021 Apr 17;11(4):1147. doi: 10.3390/ani11041147. Animals (Basel). 2021. PMID: 33920522 Free PMC article.
-
Human myotube formation is determined by MyoD-Myomixer/Myomaker axis.Sci Adv. 2020 Dec 18;6(51):eabc4062. doi: 10.1126/sciadv.abc4062. Print 2020 Dec. Sci Adv. 2020. PMID: 33355126 Free PMC article.
-
The regulatory role of Myomaker and Myomixer-Myomerger-Minion in muscle development and regeneration.Cell Mol Life Sci. 2020 Apr;77(8):1551-1569. doi: 10.1007/s00018-019-03341-9. Epub 2019 Oct 23. Cell Mol Life Sci. 2020. PMID: 31642939 Free PMC article. Review.
-
Structural Insights into Membrane Fusion Mediated by Convergent Small Fusogens.Cells. 2021 Jan 15;10(1):160. doi: 10.3390/cells10010160. Cells. 2021. PMID: 33467484 Free PMC article. Review.
References
-
- Bazzoni G, 2003. The JAM family of junctional adhesion molecules. Curr. Opin. Cell Biol. 15, 525–530. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
