A high-throughput inhibition assay to study MERS-CoV antibody interactions using image cytometry
- PMID: 30468747
- PMCID: PMC6357230
- DOI: 10.1016/j.jviromet.2018.11.009
A high-throughput inhibition assay to study MERS-CoV antibody interactions using image cytometry
Abstract
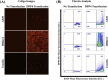
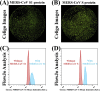
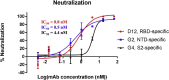
The emergence of new pathogens, such as Middle East respiratory syndrome coronavirus (MERS-CoV), poses serious challenges to global public health and highlights the urgent need for methods to rapidly identify and characterize potential therapeutic or prevention options, such as neutralizing antibodies. Spike (S) proteins are present on the surface of MERS-CoV virions and mediate viral entry. S is the primary target for MERS-CoV vaccine and antibody development, and it has become increasingly important to understand MERS-CoV antibody binding specificity and function. Commonly used serological methods like ELISA, biolayer interferometry, and flow cytometry are informative, but limited. Here, we demonstrate a high-throughput protein binding inhibition assay using image cytometry. The image cytometry-based high-throughput screening method was developed by selecting a cell type with high DPP4 expression and defining optimal seeding density and protein binding conditions. The ability of monoclonal antibodies to inhibit MERS-CoV S binding was then tested. Binding inhibition results were comparable with those described in previous literature for MERS-CoV spike monomer and showed similar patterns as neutralization results. The coefficient of variation (CV) of our cell-based assay was <10%. The proposed image cytometry method provides an efficient approach for characterizing potential therapeutic antibodies for combating MERS-CoV that compares favorably with current methods. The ability to rapidly determine direct antibody binding to host cells in a high-throughput manner can be applied to study other pathogen-antibody interactions and thus can impact future research on viral pathogens.
Keywords: Antibody binding; Antibody neutralization; Celigo; Image cytometry; Inhibition assay; MERS-CoV.
Published by Elsevier B.V.
Figures


Similar articles
-
Importance of Neutralizing Monoclonal Antibodies Targeting Multiple Antigenic Sites on the Middle East Respiratory Syndrome Coronavirus Spike Glycoprotein To Avoid Neutralization Escape.J Virol. 2018 Apr 27;92(10):e02002-17. doi: 10.1128/JVI.02002-17. Print 2018 May 15. J Virol. 2018. PMID: 29514901 Free PMC article.
-
Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants.J Virol. 2016 Dec 16;91(1):e01651-16. doi: 10.1128/JVI.01651-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795425 Free PMC article.
-
Mutations in the Spike Protein of Middle East Respiratory Syndrome Coronavirus Transmitted in Korea Increase Resistance to Antibody-Mediated Neutralization.J Virol. 2019 Jan 4;93(2):e01381-18. doi: 10.1128/JVI.01381-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30404801 Free PMC article.
-
Antibodies and vaccines against Middle East respiratory syndrome coronavirus.Emerg Microbes Infect. 2019;8(1):841-856. doi: 10.1080/22221751.2019.1624482. Emerg Microbes Infect. 2019. PMID: 31169078 Free PMC article. Review.
-
MERS-CoV spike protein: Targets for vaccines and therapeutics.Antiviral Res. 2016 Sep;133:165-77. doi: 10.1016/j.antiviral.2016.07.015. Epub 2016 Jul 26. Antiviral Res. 2016. PMID: 27468951 Free PMC article. Review.
Cited by
-
High-throughput viral microneutralization method for feline coronavirus using image cytometry.J Virol Methods. 2020 Dec;286:113979. doi: 10.1016/j.jviromet.2020.113979. Epub 2020 Sep 23. J Virol Methods. 2020. PMID: 32979406 Free PMC article.
-
High-Throughput SARS-CoV-2 Antiviral Testing Method Using the Celigo Image Cytometer.J Fluoresc. 2024 Mar;34(2):561-570. doi: 10.1007/s10895-023-03289-x. Epub 2023 Jun 13. J Fluoresc. 2024. PMID: 37310590 Free PMC article.
-
Why would a black man volunteer for a government-funded science experiment?EClinicalMedicine. 2021 Mar 19;33:100788. doi: 10.1016/j.eclinm.2021.100788. eCollection 2021 Mar. EClinicalMedicine. 2021. PMID: 33817608 Free PMC article. No abstract available.
-
Imaging Flow Cytometry in HIV Infection Research: Advantages and Opportunities.Methods Protoc. 2025 Feb 3;8(1):14. doi: 10.3390/mps8010014. Methods Protoc. 2025. PMID: 39997638 Free PMC article. Review.
-
Characterization of CAR T cell expansion and cytotoxic potential during Ex Vivo manufacturing using image-based cytometry.J Immunol Methods. 2020 Sep-Oct;484-485:112830. doi: 10.1016/j.jim.2020.112830. Epub 2020 Aug 1. J Immunol Methods. 2020. PMID: 32745474 Free PMC article.
References
-
- Beigel J.H., Voell J., Kumar P., Raviprakash K., Wu H., Jiao J.-A., Sullivan E., Luke T., Davey R.T., Jr. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect. Dis. 2018;18:410–418. - PMC - PubMed
-
- Corti D., Zhao J., Pedotti M., Simonelli L., Agnihothram S., Fett C., Fernandez-Rodriguez B., Foglierini M., Agatic G., Vanzetta F., Gopal R., Langrish C.J., Barrett N.A., Sallusto F., Baric R.S., Varani L., Zambon M., Perlman S., Lanzavecchia A. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc. Natl. Acad. Sci. 2015;112:10473–10478. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

