Selenium-binding protein 1 (SELENBP1) is a marker of mature adipocytes
- PMID: 30469030
- PMCID: PMC6249406
- DOI: 10.1016/j.redox.2018.11.004
Selenium-binding protein 1 (SELENBP1) is a marker of mature adipocytes
Abstract
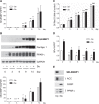
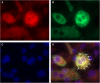
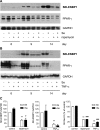
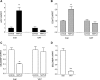
Selenium-binding protein 1 (SELENBP1) has recently been reported to catalyse the oxidation of methanethiol, an organosulfur compound produced by gut microbiota. Two of the reaction products of methanethiol oxidation, hydrogen peroxide and hydrogen sulphide, serve as signalling molecules for cell differentiation. Indeed, colonocyte differentiation has been found to be associated with SELENBP1 induction. Here, we show that SELENBP1 is induced when 3T3-L1 preadipocytes undergo terminal differentiation and maturation to adipocytes. SELENBP1 induction succeeded the up-regulation of known marker proteins of white adipocytes and the intracellular accumulation of lipids. Immunofluorescence microscopy revealed predominant cytoplasmic localisation of SELENBP1 in 3T3-L1 adipocytes, as demonstrated by co-staining with the key lipogenic enzyme, acetyl-CoA-carboxylase (ACC), located in cytosol. In differentiating 3T3-L1 cells, the mTOR inhibitor rapamycin and the pro-inflammatory cytokine tumour necrosis factor alpha (TNF-α) likewise suppressed SELENBP1 induction, adipocyte differentiation and lipid accumulation. However, lipid accumulation per se is not linked to SELENBP1 induction, as hepatic SELENBP1 was down-regulated in high fructose-fed mice despite increased lipogenesis in the liver and development of non-alcoholic fatty liver disease (NAFLD). In conclusion, SELENBP1 is a marker of cell differentiation/maturation rather than being linked to lipogenesis/lipid accumulation.
Keywords: 3T3-L1; Adipogenesis; Lipid accumulation; NAFLD; Selenium, GPx1.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Rayman M.P. Selenium and human health. Lancet. 2012;379(9822):1256–1268. - PubMed
-
- Steinbrenner H., Speckmann B., Klotz L.O. Selenoproteins: antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016;595:113–119. - PubMed
-
- Bansal M.P., Oborn C.J., Danielson K.G., Medina D. Evidence for two selenium-binding proteins distinct from glutathione peroxidase in mouse liver. Carcinogenesis. 1989;10(3):541–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

