Metformin Inhibits Migration and Invasion by Suppressing ROS Production and COX2 Expression in MDA-MB-231 Breast Cancer Cells
- PMID: 30469399
- PMCID: PMC6274682
- DOI: 10.3390/ijms19113692
Metformin Inhibits Migration and Invasion by Suppressing ROS Production and COX2 Expression in MDA-MB-231 Breast Cancer Cells
Abstract
Background: Several mechanisms of action have been proposed to explain the apparent antineoplastic functions of metformin, many of which are observed at high concentrations that may not be reflective of achievable tissue concentrations. We propose that metformin at low concentrations functions to inhibit ROS production and inflammatory signaling in breast cancer, thereby reducing metastasis.
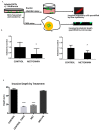
Methods: Using the highly invasive MDA-MB-231 breast carcinoma model, we ascertained the impact of metformin on cell viability by DNA content analysis and fluorescent dye exclusion. Migration and invasion assays were performed using a modified Boyden chamber assay and metastasis was ascertained using the chorioallantoic membrane (CAM) assay. PGE2 production was measured by Enzyme-Linked Immunosorbent Assay (ELISA). COX2 and ICAM1 levels were determined by flow cytometry immunoassay.
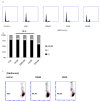
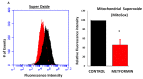
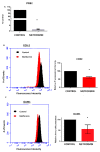
Results: Metformin acutely decreased cell viability and caused G2 cell cycle arrest only at high concentrations (10 mM). At 100 µM, however, metformin reduced ICAM1 and COX2 expression, as well as reduced PGE2 production and endogenous mitochondrial ROS production while failing to significantly impact cell viability. Consequently, metformin inhibited migration, invasion in vitro and PGE2-dependent metastasis in CAM assays.
Conclusion: At pharmacologically achievable concentrations, metformin does not drastically impact cell viability, but inhibits inflammatory signaling and metastatic progression in breast cancer cells.
Keywords: COX2; ICAM1; metastasis; metformin; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

