Long-Term Effects of Spironolactone on Kidney Function and Hyperkalemia-Associated Hospitalization in Patients with Chronic Kidney Disease
- PMID: 30469400
- PMCID: PMC6262621
- DOI: 10.3390/jcm7110459
Long-Term Effects of Spironolactone on Kidney Function and Hyperkalemia-Associated Hospitalization in Patients with Chronic Kidney Disease
Abstract
Background: Spironolactone, a non-selective mineralocorticoid receptor antagonist, can protect against cardiac fibrosis and left ventricular dysfunction, and improve endothelial dysfunction and proteinuria. However, the safety and effects of spironolactone on patient-centered cardiovascular and renal endpoints remain unclear.
Methods: We identified predialysis stage 3⁻4 chronic kidney disease (CKD) patients between 2000 and 2013 from the Longitudinal Health Insurance Database 2005 (LHID 2005). The outcomes of interest were end-stage renal disease (ESRD), major adverse cardiovascular events (MACE), hospitalization for heart failure (HHF), hyperkalemia-associated hospitalization (HKAH), all-cause mortality and cardiovascular mortality. The Fine and Gray sub-distribution hazards approach was adopted to adjust for the competing risk of death.
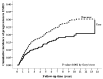
Results: After the propensity score matching, 693 patients with stage 3⁻4 CKD were spironolactone users and 1386 were nonusers. During the follow-up period, spironolactone users had a lower incidence rate for ESRD than spironolactone non-users (39.2 vs. 53.69 per 1000 person-years) and a higher incidence rate for HKAH (54.79 vs. 18.57 per 1000 person-years). The adjusted hazard ratios for ESRD of spironolactone users versus non-users were 0.66 (95% CI, 0.51⁻0.84; p value < 0.001) and 3.17 (95% CI, 2.41⁻4.17; p value < 0.001) for HKAH. A dose-response relationship was found between spironolactone use and risk of ESRD and HKAH. There were no statistical differences in MACE, HHF, all-cause mortality and cardiovascular mortality between spironolactone users and non-users.
Conclusion: Spironolactone represented a promising treatment option to retard CKD progression to ESRD amongst stage 3⁻4 CKD patients, but strategic treatments to prevent hyperkalemia should be enforced.
Keywords: chronic kidney disease (CKD); end-stage renal disease (ESRD); major adverse cardiovascular events (MACE); mortality; spironolactone.
Conflict of interest statement
There is no competing interest.
Figures





References
-
- Tomiyama C., Higa A., Dalboni M.A., Cendoroglo M., Draibe S.A., Cuppari L., Carvalho A.B., Neto E.M., Canziani M.E. The impact of traditional and nontraditional risk factors on coronary calcification in pre-dialysis patients. Nephrol. Dial. Transpl. 2006;21:2464–2471. doi: 10.1093/ndt/gfl291. - DOI - PubMed
-
- Brenner B.M., Cooper M.E., de Zeeuw D., Keane W.F., Mitch W.E., Parving H.H., Remuzzi G., Snapinn S.M., Zhang Z., Shahinfar S., et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. - DOI - PubMed
-
- Jafar T.H., Schmid C.H., Landa M., Giatras I., Toto R., Remuzzi G., Maschio G., Brenner B.M., Kamper A., Zucchelli P., et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann. Intern. Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. - DOI - PubMed
LinkOut - more resources
Full Text Sources

