Tonic ATP-mediated growth suppression in peripheral nerve glia requires arrestin-PP2 and is evaded in NF1
- PMID: 30470263
- PMCID: PMC6251093
- DOI: 10.1186/s40478-018-0635-9
Tonic ATP-mediated growth suppression in peripheral nerve glia requires arrestin-PP2 and is evaded in NF1
Abstract
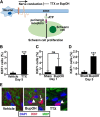
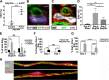
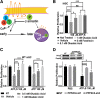
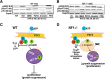
Normal Schwann cells (SCs) are quiescent in adult nerves, when ATP is released from the nerve in an activity dependent manner. We find that suppressing nerve activity in adult nerves causes SC to enter the cell cycle. In vitro, ATP activates the SC G-protein coupled receptor (GPCR) P2Y2. Downstream of P2Y2, β-arrestin-mediated signaling results in PP2-mediated de-phosphorylation of AKT, and PP2 activity is required for SC growth suppression. NF1 deficient SC show reduced growth suppression by ATP, and are resistant to the effects of β-arrestin-mediated signaling, including PP2-mediated de-phosphorylation of AKT. In patients with the disorder Neurofibromatosis type 1, NF1 mutant SCs proliferate and form SC tumors called neurofibromas. Elevating ATP levels in vivo reduced neurofibroma cell proliferation. Thus, the low proliferation characteristic of differentiated adult peripheral nerve may require ongoing, nerve activity-dependent, ATP. Additionally, we identify a mechanism through which NF1 SCs may evade growth suppression in nerve tumors.
Keywords: AKT; ATP; Arrestin; Glia; Neurofibromatosis; P2Y2; PP2A; Purinergic; Schwann.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

