The Ataxia telangiectasia-mutated and Rad3-related protein kinase regulates cellular hydrogen sulfide concentrations
- PMID: 30470507
- PMCID: PMC6925466
- DOI: 10.1016/j.dnarep.2018.11.002
The Ataxia telangiectasia-mutated and Rad3-related protein kinase regulates cellular hydrogen sulfide concentrations
Abstract
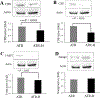
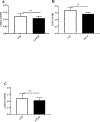
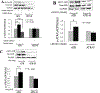
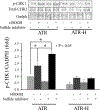
The ataxia telangiectasia-mutated and Rad3-related (ATR) serine/threonine kinase plays a central role in the repair of replication-associated DNA damage, the maintenance of S and G2/M-phase genomic stability, and the promotion of faithful mitotic chromosomal segregation. A number of stimuli activate ATR, including persistent single-stranded DNA at stalled replication folks, R loop formation, hypoxia, ultraviolet light, and oxidative stress, leading to ATR-mediated protein phosphorylation. Recently, hydrogen sulfide (H2S), an endogenous gasotransmitter, has been found to regulate multiple cellular processes through complex redox reactions under similar cell stress environments. Three enzymes synthesize H2S: cystathionine-β-synthase, cystathionine γ-lyase, and 3-mercaptopyruvate sulfurtransferase. Since H2S can under some conditions cause DNA damage, we hypothesized that ATR activity may regulate cellular H2S concentrations and H2S-syntheszing enzymes. Here we show that human colorectal cancer cells carrying biallelic knock-in hypomorphic ATR mutations have lower cellular H2S concentrations than do syngeneic ATR wild-type cells, and all three H2S-synthesizing enzymes show lower protein expression in the ATR hypomorphic mutant cells. Additionally, ATR serine 428 phosphorylation is altered by H2S donor and H2S synthesis enzyme inhibition, while the oxidative-stress induced phosphorylation of the ATR-regulated protein CHK1 on serine 345 is increased by H2S synthesis enzyme inhibition. Lastly, inhibition of H2S production potentiated oxidative stress-induced double-stranded DNA breaks in the ATR hypomorphic mutant compared to ATR wild-type cells. Our findings demonstrate that the ATR kinase regulates and is regulated by H2S.
Keywords: 3-Mercaptopyruvate sulfurtransferase; ATR; CHK1; Cystathionine γ-lyase; Cystathionine-β-synthase; H(2)S; Hydrogen sulfide; Nicotinamide; phosphoribosyltransferase.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

