The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology
- PMID: 30470559
- PMCID: PMC6380948
- DOI: 10.1016/j.biopsych.2018.09.031
The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology
Abstract
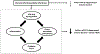
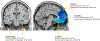
Volumetric reductions in the hippocampus and medial prefrontal cortex (mPFC) are among the most well-documented neural abnormalities in major depressive disorder (MDD). Hippocampal and mPFC structural reductions have been specifically tied to MDD illness progression markers, including greater number of major depressive episodes (MDEs), longer illness duration, and nonremission/treatment resistance. Chronic stress plays a critical role in the development of hippocampal and mPFC deficits, with some studies suggesting that these deficits occur irrespective of MDE occurrence. However, preclinical and human research also points to other stress-mediated neurotoxic processes, including enhanced inflammation and neurotransmitter disturbances, which may require the presence of an MDE and contribute to further brain structural decline as the illness advances. Specifically, hypothalamic-pituitary-adrenal axis dysfunction, enhanced inflammation and oxidative stress, and neurotransmitter abnormalities (e.g., serotonin, glutamate, gamma-aminobutyric acid) likely interact to facilitate illness progression in MDD. Congruent with stress sensitization models of MDD, with each consecutive MDE it may take lower levels of stress to trigger these neurotoxic pathways, leading to more pronounced brain volumetric reductions. Given that stress and MDD have overlapping and distinct influences on neurobiological pathways implicated in hippocampal and mPFC structural decline, further work is needed to clarify which precise mechanisms ultimately contribute to MDD development and maintenance.
Keywords: Depression; Hippocampus; Illness progression; Medial prefrontal cortex; Neuroprogression; Stress.
Copyright © 2018 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures
E.L.B has no conflicts of interest to disclose. In the past three years, M.T.T. has served as a paid consultant to NeuroCog Trials, Avanir Pharmaceuticals, and Blackthorn Therapeutics. Over the past 3 years, D.A.P. has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Posit Science, and Takeda Pharmaceuticals, for activities unrelated to the current review. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
Figures

Comment in
-
Parsing the Hippocampus in Depression: Chronic Stress, Hippocampal Volume, and Major Depressive Disorder.Biol Psychiatry. 2019 Mar 15;85(6):436-438. doi: 10.1016/j.biopsych.2019.01.011. Biol Psychiatry. 2019. PMID: 30777168 No abstract available.
References
-
- Monroe SM, Harkness K (2011): Recurrence in major depression: A conceptual analysis. Psychol Rev 118:655–674. - PubMed
-
- Videbech P, Ravnkilde B (2004): Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966. - PubMed
-
- Campbell S, Marriott M, Nahmias C, MacQueen GM (2004): Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry 161:598–607. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

