Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer
- PMID: 30470719
- PMCID: PMC6279405
- DOI: 10.1084/jem.20180818
Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer
Abstract
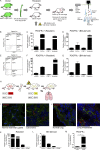
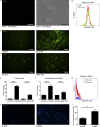
Cancer-associated fibroblasts (CAFs) are highly prominent in breast tumors, but their functional heterogeneity and origin are still largely unresolved. We report that bone marrow (BM)-derived mesenchymal stromal cells (MSCs) are recruited to primary breast tumors and to lung metastases and differentiate to a distinct subpopulation of CAFs. We show that BM-derived CAFs are functionally important for tumor growth and enhance angiogenesis via up-regulation of Clusterin. Using newly generated transgenic mice and adoptive BM transplantations, we demonstrate that BM-derived fibroblasts are a substantial source of CAFs in the tumor microenvironment. Unlike resident CAFs, BM-derived CAFs do not express PDGFRα, and their recruitment resulted in a decrease in the percentage of PDGFRα-expressing CAFs. Strikingly, decrease in PDGFRα in breast cancer patients was associated with worse prognosis, suggesting that BM-derived CAFs may have deleterious effects on survival. Therefore, PDGFRα expression distinguishes two functionally unique CAF populations in breast tumors and metastases and may have important implications for patient stratification and precision therapeutics.
© 2018 Raz et al.
Figures






Comment in
-
Deciphering cancer fibroblasts.J Exp Med. 2018 Dec 3;215(12):2967-2968. doi: 10.1084/jem.20182069. Epub 2018 Nov 23. J Exp Med. 2018. PMID: 30470718 Free PMC article.
References
-
- Anderberg C., Li H., Fredriksson L., Andrae J., Betsholtz C., Li X., Eriksson U., and Pietras K.. 2009. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 69:369–378. 10.1158/0008-5472.CAN-08-2724 - DOI - PMC - PubMed
-
- Barcellos-de-Souza P., Comito G., Pons-Segura C., Taddei M.L., Gori V., Becherucci V., Bambi F., Margheri F., Laurenzana A., Del Rosso M., and Chiarugi P.. 2016. Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-β1. Stem Cells. 34:2536–2547. 10.1002/stem.2412 - DOI - PubMed
-
- Beer T.M., Hotte S.J., Saad F., Alekseev B., Matveev V., Fléchon A., Gravis G., Joly F., Chi K.N., Malik Z., et al. . 2017. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial. Lancet Oncol. 18:1532–1542. 10.1016/S1470-2045(17)30605-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

