Amyloid precursor protein-mediated mitochondrial regulation and Alzheimer's disease
- PMID: 30471088
- PMCID: PMC6715589
- DOI: 10.1111/bph.14554
Amyloid precursor protein-mediated mitochondrial regulation and Alzheimer's disease
Abstract
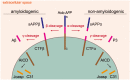
Despite clear evidence of a neuroprotective physiological role of amyloid precursor protein (APP) and its non-amyloidogenic processing products, APP has been investigated mainly in animal and cellular models of amyloid pathology in the context of Alzheimer's disease. The rare familial mutations in APP and presenilin-1/2, which sometimes drive increased amyloid β (Aβ) production, may have unduly influenced Alzheimer's disease research. APP and its cleavage products play important roles in cellular and mitochondrial metabolism, but many studies focus solely on Aβ. Mitochondrial bioenergetic metabolism is essential for neuronal function, maintenance and survival, and multiple reports indicate mitochondrial abnormalities in patients with Alzheimer's disease. In this review, we focus on mitochondrial abnormalities reported in sporadic Alzheimer's disease patients and the role of full-length APP and its non-amyloidogenic fragments, particularly soluble APPα, on mitochondrial bioenergetic metabolism. We do not review the plethora of animal and in vitro studies using mutant APP/presenilin constructs or experiments using exogenous Aβ. In doing so, we aim to invigorate research and discussion around non-amyloidogenic APP processing products and the mechanisms linking mitochondria and complex neurodegenerative disorders such as sporadic Alzheimer's disease. LINKED ARTICLES: This article is part of a themed section on Therapeutics for Dementia and Alzheimer's Disease: New Directions for Precision Medicine. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.18/issuetoc.
© 2018 The British Pharmacological Society.
Conflict of interest statement
P.v.W. heads Enlighten Imaging, which aims to develop retinal biomarkers in Alzheimer's disease.
Figures

References
-
- Aksenov MY, Tucker HM, Nair P, Aksenova MV, Butterfield DA, Estus S et al (1999). The expression of several mitochondrial and nuclear genes encoding the subunits of electron transport chain enzyme complexes, cytochrome c oxidase, and NADH dehydrogenase, in different brain regions in Alzheimer's disease. Neurochem Res 24: 767–774. - PubMed
-
- Ankarcrona M, Hultenby K (2002). Presenilin‐1 is located in rat mitochondria. Biochem Biophys Res Commun 295: 766–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

