Effect of hormone modulations on donor-derived spermatogenesis or colonization after syngeneic and xenotransplantation in mice
- PMID: 30471208
- PMCID: PMC6422689
- DOI: 10.1111/andr.12571
Effect of hormone modulations on donor-derived spermatogenesis or colonization after syngeneic and xenotransplantation in mice
Abstract
Background: Cytotoxic cancer treatments, such as irradiation, can cause permanent sterility in male mammals owing to the loss of spermatogonial stem cells. In animal models, spermatogenesis could be restored from transplanted spermatogonial stem cells. Previously, we showed that transient suppression of FSH, LH, and testosterone in the recipient with a gonadotropin-releasing hormone antagonist (GnRH-ant), given immediately after irradiation, enhanced spermatogenesis from transplanted spermatogonial stem cells in mice and monkeys.
Objectives: To explore improvements in the preparation of the recipient for efficient and reliable spermatogenic recovery from spermatogonial stem cell transplantation, so that it can be used effectively in clinical practice.
Materials and methods: In mouse recipients, we evaluated the effects of hormone suppression given after germ cell depletion was complete, which is a more clinically relevant model, and also the importance of total androgen ablation and maintenance of FSH levels. Three regimens, GnRH-ant, GnRH-ant plus flutamide (androgen receptor antagonist), and GnRH-ant plus FSH, were administered prior to and around the time of transplantation of testis cells from immature mice or from prepubertal monkeys.
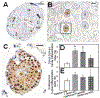
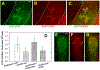
Results: Treatment with GnRH-ant resulted in a fourfold increase in spermatogenic recovery from GFP-marked transplanted mouse cells. Total androgen ablation with the addition of flutamide, started two weeks before transplantation, did not further enhance recovery. Surprisingly, FSH supplementation, started around the time of transplantation, actually reduced spermatogenic recovery from transplanted spermatogonial stem cells in GnRH-ant-treated mice. When prepubertal monkey testicular cells were transplanted into nude mice that were given the same hormone treatments, the numbers of donor-derived colonies were independent of hormone treatment.
Discussion and conclusion: The enhancements in spermatogenic recovery may only occur when syngeneic or closely related donor-recipient pairs are used. These results are useful in further investigations in choosing a hormone suppression regimen in combination with spermatogonial transplantation as a treatment to restore fertility in primates after cytotoxic therapy.
Keywords: GnRH-antagonist; irradiation; spermatogonia; transplantation.
© 2018 American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
DISCLOSURES
The authors have no conflicting financial interests.
Figures
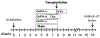


Comment in
-
Uro-Science.J Urol. 2022 Feb;207(2):460-461. doi: 10.1097/JU.0000000000002305. Epub 2021 Nov 12. J Urol. 2022. PMID: 34766838 No abstract available.
Similar articles
-
Hormone suppression with GnRH antagonist promotes spermatogenic recovery from transplanted spermatogonial stem cells in irradiated cynomolgus monkeys.Andrology. 2013 Nov;1(6):886-98. doi: 10.1111/j.2047-2927.2013.00126.x. Epub 2013 Sep 30. Andrology. 2013. PMID: 24124124 Free PMC article.
-
Restoration of functional sperm production in irradiated pubertal rhesus monkeys by spermatogonial stem cell transplantation.Andrology. 2020 Sep;8(5):1428-1441. doi: 10.1111/andr.12807. Epub 2020 May 18. Andrology. 2020. PMID: 32351003 Free PMC article.
-
Postpubertal spermatogonial stem cell transplantation restores functional sperm production in rhesus monkeys irradiated before and after puberty.Andrology. 2021 Sep;9(5):1603-1616. doi: 10.1111/andr.13033. Epub 2021 Jul 7. Andrology. 2021. PMID: 33960147 Free PMC article.
-
Spermatogonial Stem Cell Culture in Oncofertility.Urol Clin North Am. 2020 May;47(2):227-244. doi: 10.1016/j.ucl.2020.01.001. Urol Clin North Am. 2020. PMID: 32272995 Free PMC article. Review.
-
Spermatogonial stem cell autotransplantation and germline genomic editing: a future cure for spermatogenic failure and prevention of transmission of genomic diseases.Hum Reprod Update. 2016 Sep;22(5):561-73. doi: 10.1093/humupd/dmw017. Epub 2016 May 30. Hum Reprod Update. 2016. PMID: 27240817 Free PMC article. Review.
Cited by
-
DAZL Knockout Pigs as Recipients for Spermatogonial Stem Cell Transplantation.Cells. 2023 Nov 6;12(21):2582. doi: 10.3390/cells12212582. Cells. 2023. PMID: 37947660 Free PMC article.
-
Approaches and Technologies in Male Fertility Preservation.Int J Mol Sci. 2020 Jul 31;21(15):5471. doi: 10.3390/ijms21155471. Int J Mol Sci. 2020. PMID: 32751826 Free PMC article. Review.
-
Models and Methods for Evaluating Regeneration of Spermatogenesis After Cytotoxic Treatments.Methods Mol Biol. 2023;2656:239-260. doi: 10.1007/978-1-0716-3139-3_14. Methods Mol Biol. 2023. PMID: 37249876
References
-
- Albuquerque AV, Almeida FR, Weng CC, Shetty G, Meistrich ML & Chiarini-Garcia H. (2013) Spermatogonial behavior in rats during radiation-induced arrest and recovery after hormone suppression. Reproduction 146, 363–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

