Keratocytes Generate High Integrin Tension at the Trailing Edge to Mediate Rear De-adhesion during Rapid Cell Migration
- PMID: 30472533
- PMCID: PMC6257914
- DOI: 10.1016/j.isci.2018.11.016
Keratocytes Generate High Integrin Tension at the Trailing Edge to Mediate Rear De-adhesion during Rapid Cell Migration
Abstract
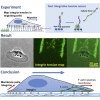
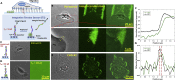
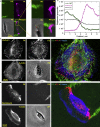
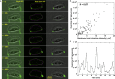
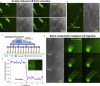
Rapid cell migration requires efficient rear de-adhesion. It remains undetermined whether cells mechanically detach or biochemically disassemble integrin-mediated rear adhesion sites in highly motile cells such as keratocytes. Using molecular tension sensor, we calibrated and mapped integrin tension in migrating keratocytes. Our experiments revealed that high-level integrin tension abbreviated as HIT, in the range of 50-100 pN (piconewton) and capable of rupturing integrin-ligand bonds, is exclusively and narrowly generated at cell rear margin during cell migration. Co-imaging of HIT and focal adhesions (FAs) shows that HIT is produced to mechanically peel off FAs that lag behind, and HIT intensity is correlated with the local cell retraction rate. High-level molecular tension was also consistently generated at the cell margin during artificially induced cell front retraction and during keratocyte migration mediated by biotin-streptavidin bonds. Collectively, these experiments provide direct evidence showing that migrating keratocytes concentrate force at the cell rear margin to mediate rear de-adhesion.
Keywords: Biomechanics; Biophysics; Functional Aspects of Cell Biology.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Carragher N.O., Frame M.C. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. - PubMed
-
- Even-Ram S., Doyle A.D., Conti M.A., Matsumoto K., Adelstein R.S., Yamada K.M. Myosin IIA regulates cell motility and actomyosin microtubule crosstalk. Nat. Cell Biol. 2007;9:299–309. - PubMed
-
- Franco S.J., Huttenlocher A. Regulating cell migration: calpains make the cut. J. Cell Sci. 2005;118:3829–3838. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

